Urinary tract infection (UTI) is a common condition in women. There is an increased concern on reduction of bacterial susceptibility resulting from wrongly prescribing antimicrobials. This paper summarizes the recommendations of four Brazilian medical societies (SBI – Brazilian Society of Infectious Diseases, FEBRASGO – Brazilian Federation of Gynecology and Obstetrics Associations, SBU – Brazilian Society of Urology, and SBPC/ML – Brazilian Society of Clinical Pathology/Laboratory Medicine) on the management of urinary tract infection in women.
Asymptomatic bacteriuria should be screened at least twice during pregnancy (early and in the 3rd trimester). All cases of significant bacteriuria (≥105CFU/mL in middle stream sample) should be treated with antimicrobials considering safety and susceptibility profile. In women with typical symptoms of cystitis, dipsticks are not necessary for diagnosis. Urine cultures should be collected in pregnant women, recurrent UTI, atypical cases, and if there is suspicion of pyelonephritis. First line antimicrobials for cystitis are fosfomycin trometamol in a single dose and nitrofurantoin, 100mg every 6hours for five days. Second line drugs are cefuroxime or amoxicillin-clavulanate for seven days. During pregnancy, amoxicillin and other cephalosporins may be used, but with a higher chance of therapeutic failure.
In recurrent UTI, all episodes should be confirmed by urine culture. Treatment should be initiated only after urine sampling and with the same regimens indicated for isolated episodes. Prophylaxis options of recurrent UTI are behavioral measures, non-antimicrobial and antimicrobial prophylaxis. Vaginal estrogens may be recommended for postmenopausal women. Other non-antimicrobial prophylaxis, including cranberry and immunoprophylaxis, have weak evidence supporting their use. Antimicrobial prophylaxis may be offered as a continuous or postcoital scheme. In pregnant women, options are cephalexin, 250–500mg and nitrofurantoin, 100mg (contraindicated after 37 weeks of pregnancy). Nonpregnant women may use fosfomycin trometamol, 3g every 10 days, or nitrofurantoin, 100mg (continuous or postcoital).
Urinary tract infection (UTI) is a common condition. Annually, it affects more than 10% of women, and more than 50% of women will have at least one symptomatic episode during their lifetime.1 After the first episode of UTI, 24% of young women will recur within six months, and 2% to 5% will develop recurrent UTI (rUTI).2,3
There is an increasing concern about the development of bacterial resistance caused by the use, generally inappropriate, of antibiotics. The empirical use of broad spectrum antimicrobials for mild infections contributes to the selection of increasingly resistant strains – extended-spectrum β-lactamase (ESBL) enterobacteria, most often Escherichia coli, Klebsiella spp., Enterobacter spp., and Klebsiella pneumoniae carbapenemase (KPC) – limiting therapeutic options in severe cases with systemic infection.4
This text addresses evidence-based recommendations on management of asymptomatic bacteriuria and lower UTI in nonpregnant and pregnant women.
We consulted the following Guidelines, Recommendations and Protocols of the following Societies/Associations and Government Agencies:
- –
FEBRASGO (Brazilian Federation of Gynecology and Obstetrics Associations)5,6
- –
IDSA (Infectious Diseases Society of America)7,8
- –
EAU (European Association of Urology)4
- –
USPSTF (U.S. Preventive Services Task Force)9
- –
ACOG (American College of Obstetricians and Gynecologists)10,11
- –
NICE (National Institute for Health and Care Excellence – UK)12
- –
Brazil, Ministry of Health (MS)13
- –
AUA (American Urologic Association), 2019 – with CUA (Canadian Urological Association) and SUFU (Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction)14
Asymptomatic bacteriuria (ASB) is the presence of one or more species of bacteria in significant amount [≥105 colony-forming units (CFU)/mL in a midstream urine sample or ≥102CFU/mL in a catheterized specimen], regardless of the presence of pyuria, in the absence of signs or symptoms of UTI.8
ASB is a common condition in healthy women, affecting 1% to 5% during reproductive years and 2.8% to 8.6% at postmenopausal age. In institutionalized elderly women, the prevalence is as high as 50%.5 The condition is benign, and randomized studies have shown that, except for pregnant women, the use of antibiotics has no benefits when compared to non-treatment; in addition, there is an increased risk of bacterial resistance and Clostridium difficile infection.5
Urine specimen collection must follow correct technique for reliable results.15 The sample must be collected in the laboratory, using, whenever possible, the first void urine. Otherwise, urine should be retained in the bladder for at least 2h before collection in order to reduce the occurrence of false negative results. Increased water intake for bladder repletion is not recommended, because excess fluid dilutes urine, decreasing the colony count or yielding false negative results.15
General recommendations on ASB8- 1.
Do not request urine culture for adult patients without urinary symptoms, except in two situations:
- a.
Pregnant women;
- b.
Before an invasive urological procedure.
- a.
- 2.
Do not request urine culture in asymptomatic patients with alteration of urine color, clarity or odor.
- 3.
Do not request post-treatment urine cultures in asymptomatic patients, except for pregnant women.
- 4.
Do not treat ASB, even if the isolated uropathogen is multidrug resistant, in the following cases:
- a.
Elderly women with cognitive dysfunction;
- b.
Non-pregnant healthy young women;
- c.
Postmenopausal women;
- d.
Diabetic patients;
- e.
Patients with indwelling urethral catheter;
- f.
Patients with spinal cord injury;
- g.
Patients who have received kidney or other solid organ transplant;
- h.
Elective non-urological surgery (e.g., orthopedic implants);
- i.
Prior to urological device implantation (only perform standard perioperative antimicrobial prophylaxis prior the procedure);
- j.
Pediatric patients.
- a.
In some situations, it is uncertain if there is a recommendation for or against screening or treating ASB: high-risk neutropenia (absolute neutrophil count <100/mm3, ≥7 days’ duration following chemotherapy) and at the time of indwelling catheter removal (may reduce the risk of UTI in some patients).
Asymptomatic bacteriuria in pregnancyEpidemiologyThe prevalence of ASB during pregnancy is the same as that in non-pregnant women (2%–10%). ASB progression to symptomatic UTI occurs in approximately 25% of patients. ASB is associated with increased perinatal morbidity (prematurity and low birth weight). Untreated ASB is related to pyelonephritis in up to 40% of pregnancies; however, with treatment, this rate drops to 3%.16,17
EtiologyProximity of anus to peri-urethra in women favors infection with enterobacteria. More than 80% of bacterial infections are caused by E. coli, followed by other Gram-negative strains (Klebsiella spp., Enterobacter spp., Proteus mirabilis). The most common Gram-positive bacteria are Staphylococcus saprophyticus and Streptococcus agalactiae (Group B Streptococcus).18
Asymptomatic bacteriuria in pregnancy – recommendations- 1.
All pregnant women should be screened for ASB in early prenatal care and at the beginning of third trimester. In cases of increased risk of infection (e.g., diabetes mellitus), consider more frequent screening.
- 2.
ASB is diagnosed by urine culture with significant bacteriuria (≥105 CFU/mL in a midstream urine sample or ≥102CFU/mL in a sample collected by urethral catheterization).
- 3.
All pregnant women with ASB should be treated with antibiotics.
- 4.
The choice of antimicrobial should be based on the antibiotic sensitivity profile and its safety during pregnancy (FDA pregnancy risk category).
- 5.
Recommended treatment options for ASB in pregnancy are amoxicillin, cephalexin, cefuroxime, fosfomycin trometamol, and nitrofurantoin (Table 1).
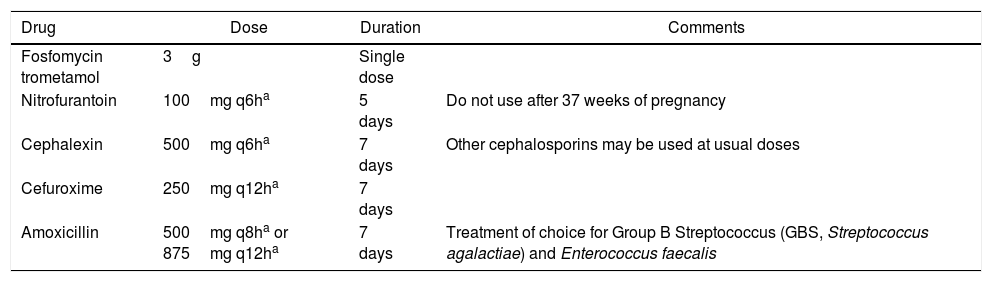
Table 1.Recommended antibiotics for treatment of ASB in pregnancy.
Drug Dose Duration Comments Fosfomycin trometamol 3g Single dose Nitrofurantoin 100mg q6ha 5 days Do not use after 37 weeks of pregnancy Cephalexin 500mg q6ha 7 days Other cephalosporins may be used at usual doses Cefuroxime 250mg q12ha 7 days Amoxicillin 500mg q8ha or 875mg q12ha 7 days Treatment of choice for Group B Streptococcus (GBS, Streptococcus agalactiae) and Enterococcus faecalis - 6.
Therapy duration is five days for nitrofurantoin, seven days for beta-lactams, or a single dose of fosfomycin trometamol.
- 7.
A control urine culture should be done 1–2 weeks after the end of treatment and, if positive, should be treated as stated above.
- 8.
Antimicrobial prophylaxis must be carried out until late pregnancy after the second episode of ASB or, if there is history of rUTI, after the first episode of ASB.
- 9.
The antibiotic may be used in the postcoital regimen in those patients who have UTIs related to sexual activity, or continuously (at bedtime).
- 10.
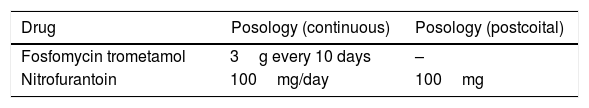
Antibiotics recommended for prophylaxis are nitrofurantoin (do not use after 37 weeks of gestation) and cephalexin (Table 2).
Table 2.Recommended regimens for UTI antimicrobial prophylaxis in pregnancy.a
Drug Dose Comments Nitrofurantoin 100mg Do not use after 37 weeks of pregnancy Cephalexin 250–500mg
Uncomplicated cystitis is defined as an acute, non-recurrent bladder infection in a healthy, nonpregnant woman without anatomical or functional abnormality of the urinary tract.19
EtiologyMost cystitis in women are caused by enterobacteria. The ARESC study, which assessed the etiology and bacterial susceptibility profile in Brazil and nine European countries between 2003 and 2006, showed that approximately three quarters of cystitis in Brazil were caused by E. coli; Gram-positive species were identified in about 5% of the cultures.20
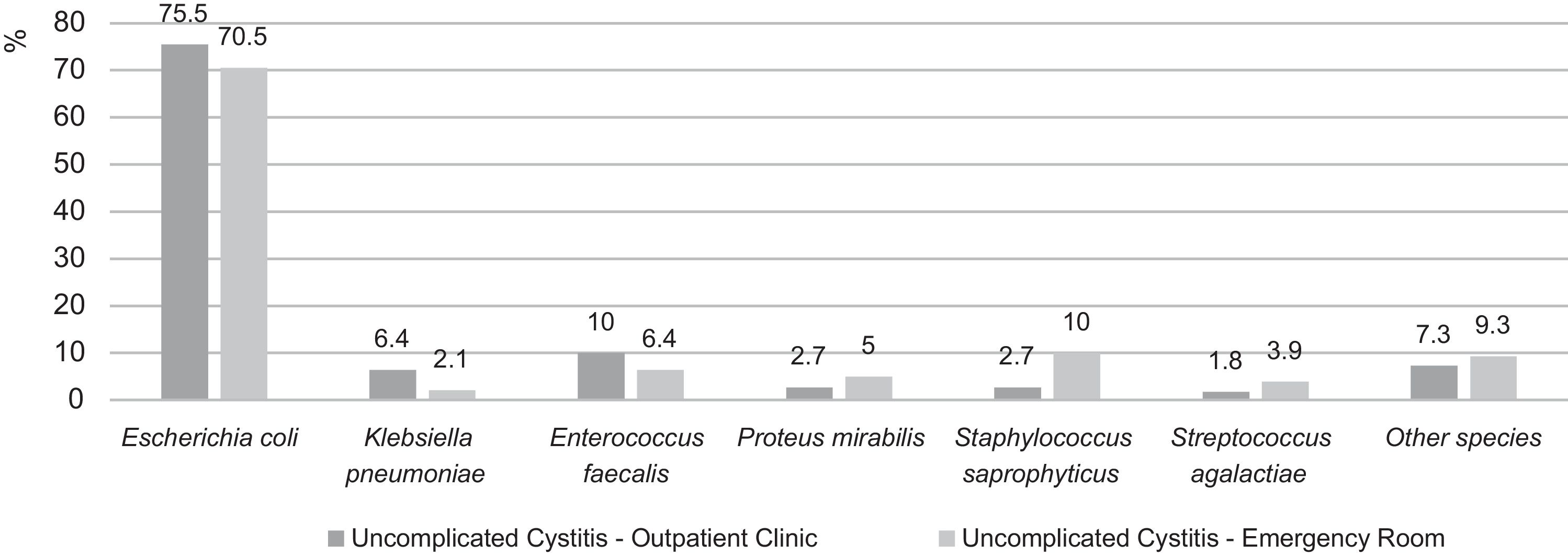
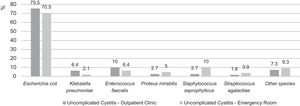
Recently (2007–2012), Hisano et al. analyzed culture results of women with uncomplicated cystitis from a quaternary hospital in São Paulo.21 As in the ARESC study, E. coli was the most prevalent species – followed, in outpatients, by E. faecalis and, in emergency room patients, by S. saprophyticus (Fig. 1).
Etiology distribution of uncomplicated cystitis in outpatients and emergency room patients in a quaternary hospital of São Paulo (2007–2012). Adapted from Hisano et al.21
Usually, the patient presents with acute onset of dysuria, increased urinary frequency, urinary urgency, suprapubic pain/tenderness, and hematuria.22
The differential diagnosis for uncomplicated cystitis includes pyelonephritis (fever, chills, flank pain, costovertebral angle tenderness, nausea/vomiting) and urethritis by Neisseria gonorrhoeae, Chlamydia trachomatis or Ureaplasma spp. Vulvovaginal infections such as candidiasis or genital herpes and irritative/allergic vulvitis may cause dysuria and should be ruled out.11,14,22
The appearance of urine (color, odor, or transparency) should never be used as isolated criteria for diagnosis of UTI or start antimicrobial therapy.23
DiagnosisClinical presentation has high sensitivity and high specificity for diagnosis of acute cystitis. Presence of dysuria, frequency, hematuria, nocturia, and urgency all increase the probability of UTI (likelihood ratio – LR>1), while presence of vaginal discharge decreases the probability of UTI (LR<1).24 In women with one or more symptoms of UTI, the probability of infection is approximately 50%; if the patient has dysuria and frequency without vaginal discharge or irritation, the probability of UTI increases to more than 90%.25
In patients with typical presentation, urinary dipstick minimally increases diagnostic accuracy; additionally, a negative result does not exclude infection.4,22 Pyuria is a nonspecific finding, so it is not sufficient to confirm a diagnosis of a UTI in the absence of symptoms.6,7
The gold standard for the diagnosis of UTI is a positive urine culture. Despite not being indicated in uncomplicated cystitis, urine culture and antimicrobial susceptibility test should be performed in pregnant women, women with suspected acute pyelonephritis, and in recurrent infection (due to higher risk of bacterial resistance). Urine culture is also recommended in women who present with atypical symptoms, therapeutic failures (defined as absence of clinical improvement after 48h of treatment), and UTI recurrence within four weeks of the end of treatment.4,6,10
TreatmentPhenazopyridine, 200mg 3 times daily for up to 48h, may be used to relieve moderate to severe dysuria. Choice of antimicrobial therapy should be guided by spectrum and local susceptibility patterns of the pathogens, tolerability and adverse effects, risk of bacterial resistance, costs, and availability.4,6
Nitrofurantoin is active against E. coli (∼90% of strains), Enterococcus spp., S. aureus, S. saprophyticus, and Strep B (S. agalactiae), while Proteus spp. and Pseudomonas spp. are intrinsically resistant to this antibiotic. Fosfomycin trometamol is active against E. coli (including ESBL-producing strains), Enterococcus spp., S. aureus and S. epidermidis. Susceptibility studies have data showing limited activity of fosfomycin against S. saprophyticus.26,27
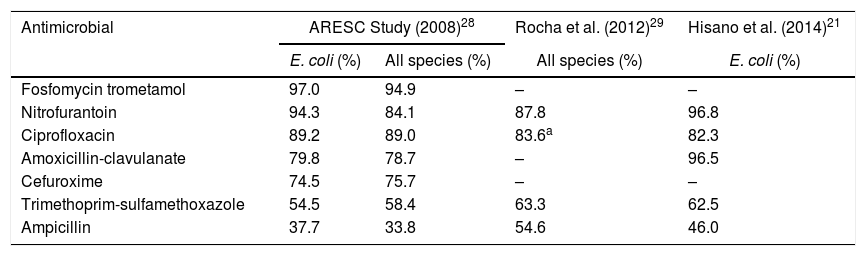
In ARESC study, fosfomycin trometamol and nitrofurantoin were the most active drugs against bacteria isolated from women with cystitis (Table 3).28 Among E. coli, susceptibility was >90% for both drugs (97.0% and 94.3%, respectively). However, overall susceptibility to nitrofurantoin was lower (84.1%). The highest resistance rates were for ampicillin and trimethoprim-sulfamethoxazole (TMP-SMX).28
Susceptibility of uropathogens for antimicrobials in Brazil.
| Antimicrobial | ARESC Study (2008)28 | Rocha et al. (2012)29 | Hisano et al. (2014)21 | |
|---|---|---|---|---|
| E. coli (%) | All species (%) | All species (%) | E. coli (%) | |
| Fosfomycin trometamol | 97.0 | 94.9 | – | – |
| Nitrofurantoin | 94.3 | 84.1 | 87.8 | 96.8 |
| Ciprofloxacin | 89.2 | 89.0 | 83.6a | 82.3 |
| Amoxicillin-clavulanate | 79.8 | 78.7 | – | 96.5 |
| Cefuroxime | 74.5 | 75.7 | – | – |
| Trimethoprim-sulfamethoxazole | 54.5 | 58.4 | 63.3 | 62.5 |
| Ampicillin | 37.7 | 33.8 | 54.6 | 46.0 |
An epidemiological study carried out in the city of Curitiba, Brazil evaluated urine cultures from outpatients between May and December 2009.29 Urine cultures with ≥105CFU/mL were considered positive and submitted to antimicrobial susceptibility testing. Exclusion criteria included fungi, mixed cultures, age <13 years old, and inpatient samples. In patients with more than one urine culture, only the first specimen was considered for analysis.29
From a total of 67,650 urine cultures, 12,567 were positive. After application of the exclusion criteria, 2769 samples were excluded, remaining 9798 urine cultures (8700 from female patients). Nitrofurantoin and fluoroquinolones were the most active oral antimicrobials; fosfomycin trometamol was not tested (Table 3).29
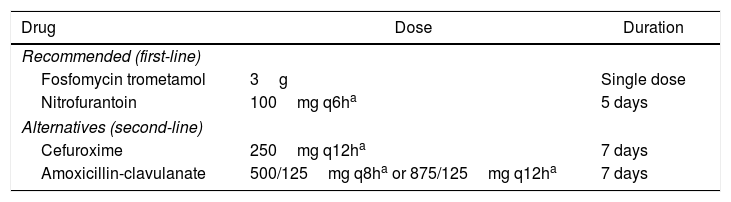
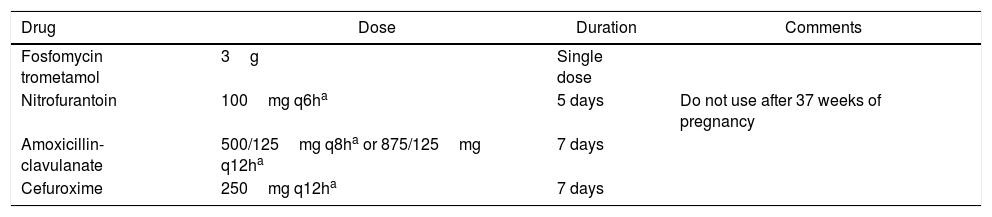
Antimicrobial treatmentThe recommended antibiotics for uncomplicated cystitis in women are fosfomycin trometamol (3g orally, in a single dose) and nitrofurantoin (100mg orally, every 6h, for five days). These drugs have unique mechanisms of action and low resistance rates. They also present high urinary concentrations and are active against ESBL-producing bacteria.30
Second-line alternatives are cefuroxime, a second-generation cephalosporin, and amoxicillin-clavulanate (Table 4).
Recommended regimens for uncomplicated cystitis in nonpregnant women.
Fluoroquinolones (FQ – norfloxacin, ciprofloxacin, levofloxacin) are not recommended in uncomplicated cystitis due to reduced uropathogen susceptibility and development of bacterial resistance. In addition, these drugs may cause severe and debilitating adverse effects, including tendinitis/tendon rupture, muscle weakness, peripheral neuropathy, autonomic and cognitive dysfunction, seizure, dementia, psychiatric disorders, rupture of aortic aneurysm, arrhythmias and dysglycemia (changes in glucose metabolism leading to hypo- or hyperglycemia) – conditions defined by the FDA in 2016 as Fluoroquinolone-Associated Disability (FQAD).31
Similarly, in 2019, the European Medicines Agency (EMA) issued recommendations restricting FQ use due to the risk of disabling and potentially permanent side effects.32 Restrictions apply, among other things, to treating mild or moderate lower UTI and preventing rUTI. It also recommends special caution in elderly patients, patients with kidney disease, transplant recipients, and those using corticosteroids, due to higher risk of tendon injury.32
In conclusion, FQ must not be used to treat cystitis, unless no other options are available.4,6
Follow upIf symptoms resolve (clinical cure), there is no indication for urine culture after treatment – except in pregnant women.14
Cystitis in pregnancyCystitis affects 2% of pregnancies and are often preceded by untreated ASB.33,34
As in nonpregnant women, the diagnosis of cystitis is based on clinical presentation. Specifically, the complaint of dysuria must be valued once urinary frequency and urgency may be present during pregnancy in the absence of infection.35,36
All patients must receive antimicrobial treatment. Whenever possible, FDA category B drugs should be used.4,5 Besides regimens indicated for nonpregnant women, amoxicillin or first-generation cephalosporins (e.g., cephalexin) may be used as alternatives – but with a higher chance of therapeutic failure (Table 5).4,6,19
Urine culture must be collected prior to antimicrobial use and 1–2 weeks after the end of treatment.
Antimicrobial prophylaxis must be taken until delivery after the second episode of cystitis or, if there is history of rUTI, after the first episode. Indicated regimens are the same used for prophylaxis after ASB (Table 2).4,5
In cases of severe dysuria, phenazopyridine (FDA category B) may be used at a dose of 200mg every 8h for up to 48h.37
Uncomplicated cystitis in women and during pregnancy – Recommendations- 1.
Typical cases of cystitis do not require further tests to confirm the diagnosis. Patients with dysuria and frequency without vaginal discharge or irritation have >90% chance of UTI.
- 2.
Urine culture should be collected prior to treatment, including cases with diagnostic doubt or suspected pyelonephritis.
- 3.
The first-line antimicrobials for cystitis are nitrofurantoin and fosfomycin trometamol; alternatives (second-line drugs) are cefuroxime and amoxicillin-clavulanate. Cephalosporins or amoxicillin may be used, but with a higher chance of therapeutic failure.
- 4.
Antimicrobial prophylaxis must be taken until delivery after the second episode of cystitis or, if there is a history of rUTI, after the first episode.
- 5.
Nitrofurantoin or cephalexin, in continued or postcoital regimens, are drugs of choice for antimicrobial prophylaxis during pregnancy.
- 6.
Fluoroquinolones (norfloxacin, ciprofloxacin, levofloxacin) must not be used to treat uncomplicated cystitis in women or during pregnancy.
Recurrent urinary tract infection (rUTI) is defined as recurrence of at least three UTIs in one year or at least two episodes in six months.4 In each episode the patient should have acute onset symptoms and bacteriuria ≥102CFU/mL in a midstream void urine sample.14
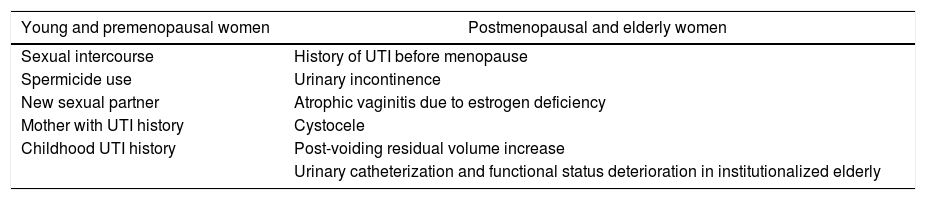
Epidemiology and risk factorsAn estimated 25% of women who have had an UTI will develop a new episode within six months.38 Several risk factors for recurrence have been identified in premenopausal young women and in elderly or postmenopausal women (Table 6).4
Age-related risk factors associated with rUTI in women.
| Young and premenopausal women | Postmenopausal and elderly women |
|---|---|
| Sexual intercourse | History of UTI before menopause |
| Spermicide use | Urinary incontinence |
| New sexual partner | Atrophic vaginitis due to estrogen deficiency |
| Mother with UTI history | Cystocele |
| Childhood UTI history | Post-voiding residual volume increase |
| Urinary catheterization and functional status deterioration in institutionalized elderly |
Adapted from EAU Guidelines.4
The proportion of UTI caused by non-E. coli species is higher in rUTI in comparison to sporadic infections. There is also an increased frequency of resistant uropathogens in recurrent episodes.39
EvaluationDiagnostic evaluation requires a comprehensive patient history and physical examination. Urinary tract exams (cystoscopy, kidney and bladder ultrasound) are not required in women with rUTI – except when associated conditions such as nephrolithiasis, obstruction, or urothelial cancer are suspected.4,14
All episodes of cystitis must be confirmed by urine culture. If the initial sample is suspected of contamination, consider collecting a new specimen – if necessary (such as in patients with urinary incontinence), by urethral catheterization.14
Periodic urine cultures are not recommended in asymptomatic patients, and antibiotics should not be prescribed in cases of bacteriuria (“don’t screen, don’t treat”).14
TreatmentAcute episodes must be treated empirically – with fosfomycin trometamol and nitrofurantoin as first choice –, considering results of previous cultures, recent use of antibiotics and the local bacterial resistance pattern. Short-term regimens (≤7 days) should be preferred.4,14
Infections caused by bacteria resistant to oral antibiotics should be treated with parenteral antibiotics for the shortest time possible (ideally, less than seven days).14
Prophylaxis of new episodes of UTIStrategies for prophylaxis of new episodes of UTI include behavioral measures, non-antimicrobial prophylaxis and antimicrobial prophylaxis. Risk factors for rUTI must be identified and treated – for example, changing the contraceptive method (stopping spermicide use) and treating the cause of significant residual urine.4,14
Behavioral measuresPatients should be counseled on behavioral changes that may reduce the risk of UTI (Table 7).4,6,14,19 Although these measures have not shown reduction in the risk of rUTI in well-designed prospective studies, it is reasonable to offer them to patients because of their low risk and their potential for effectiveness.19
Behavioral modifications for prevention of rUTI.
| Wiping from front to back after defecation |
| Liberal fluid intake |
| Do not postpone urination |
| Postcoital voiding |
| Avoid vaginal douching |
| Do not wear occlusive underwear/clothes |
Adapted from EAU Guidelines.4
The use of vaginal (but not oral) estrogen reduces the risk of rUTI and may be offered to all postmenopausal patients.4,6,14 The options currently available in Brazil are estriol (1mg/g vaginal cream) and promestriene (10mg/g vaginal cream and 10mg vaginal capsules). The treatment with estriol is started with 0.5mg (1 full applicator) daily for two weeks followed by the same dose twice a week.40,41 The initial dose for promestriene is 10mg (1 full applicator or one vaginal capsule) for 20 consecutive days, then two times/week.42
Estrogen by vaginal route has low systemic absorption and does not require association with progestogens for endometrial protection.14,43 Treatment may be continued as needed with no time limit.20 There is no safety data for vaginal estrogen use in patients at high risk for endometrial cancer. Cases of breast cancer must be individualized, with preference to promestriene.36
CranberryThe use of cranberry in rUTI is based on the presence of proanthocyanidin A, which prevents bacterial fimbria adherence to the urothelium. Clinical studies with cranberry use different doses and presentations, including juices, capsules, and tablets, making it difficult to compare efficacy results.4,6,14
There is no consensus on the indication of cranberry as rUTI prophylaxis: there are guidelines that advocate its use (without specifying which formulation),14 but others that do not recommend it due to the lack of consistent efficacy results.4,6
Because of the lack of solid evidence of clinical benefit, the use of cranberry for rUTI prophylaxis in women is not strongly recommended.
ImmunoprophylaxisOM-89 is an oral immunotherapy made with fragments of 18 strains of E. coli. This immunoactive compound is administered in capsules for 90 consecutive days.4
Immunoprophylaxis is not a consensus in the literature. This medication is recommended for rUTI prevention in women by EAU and FEBRASGO, but not by AUA/CUA/SUFU guidelines.4,6,14
Although there are some randomized clinical trials using OM-89 with positive results, we consider that there is no scientific evidence enough to strongly recommend the use of OM-89 in patients with rUTI.
Other modalities of non-antimicrobial prophylaxisAntimicrobial prophylaxisAfter discussing risks, benefits, and alternatives, women of all ages may be offered antimicrobial rUTI prophylaxis.3,14,25
Antimicrobials are effective in reducing rUTI, but their disadvantages include the risk of adverse effects and the development of bacterial resistance.4,6,14
There are two strategies for the prophylactic use of antimicrobials: continuous or postcoital administration.4,6,14 The continuous regimen consists of daily administration at bedtime. For women that notice that their UTI episodes are related to sexual activity, the antimicrobial is taken before or after sexual intercourse. This strategy has the advantage of less exposure to antibiotics and fewer side effects.14
Prophylaxis may be given from six months to one year, emphasizing that the prophylactic effect is only observed during use.4,6,14
Recommended regimens are listed in Table 8; there is no advantage in periodically changing the antibiotic.12,17
Although FQ have been used for prophylaxis, they are no longer recommended because the risks of their use in uncomplicated UTIs outweigh the benefits.25
Prolonged use of nitrofurantoin (>14 days) may cause pneumonitis; the risk increases with age and is higher in women with renal dysfunction.44–46
Recurrent urinary tract infection – recommendations- 1.
Patients with rUTI (two or more episodes of UTI in six months or three or more in one year) should be evaluated with a comprehensive history and physical examination.
- 2.
All suspected rUTI episodes must be confirmed by urine culture.
- 3.
Routine workup of the urinary tract (e.g. cystoscopy, urinary tract ultrasound) is not recommended in cases of rUTI without risk factors.
- 4.
Periodic urine cultures are not recommended in asymptomatic patients, and antibiotics should not be prescribed in cases of bacteriuria (“don’t screen, don’t treat”).
- 5.
In acute episodes in rUTI patients, empirical treatment must be initiated with the usual cystitis regimens after urine culture sampling (Table 5).
- 6.Acute episodes
caused by bacteria resistant to oral antibiotics should be treated with parenteral antibiotics for the shortest time possible.
- 7.
Risk factors for rUTI must be identified and treated.
- 8.
Behavioral measures should be recommended to all patients.
- 9.
Vaginal estrogens (estriol or promestriene), if not contraindicated, may be offered to postmenopausal women. There is no need to associate progestogens for endometrial protection.
- 10.
There is no scientific evidence enough to recommend cranberry or OM-89 for rUTI prophylaxis and their use may be discussed individually with each patient.
- 11.
d-Mannose, intravesical instillation, methenamine, probiotics, herbal therapies and biofeedback are not recommended as prophylaxis.
- 12.
After discussion of risks and benefits, antimicrobial prophylaxis with either fosfomycin trometamol or nitrofurantoin may be offered on a continuous or postcoital regimen for six to 12 months (Table 8).
Patricia de Rossi: speaker (Zambon)
Sergio Cimerman: no conflict of interest
José Carlos Truzzi: speaker (Zambon)
Clovis Arns da Cunha: consultation fee/participation in advisory board, clinical research, speaker: AstraZeneca, Bayer, Cerexa Inc., Eurofarma, Janssen, MSD, Novartis, Pfizer/Wyeth, Sanofi-Aventis, Zambon
Rosiane Mattar: no conflict of interest
Marinês Dalla Valle Martino: no conflict of interest
Maurício Hachul: no conflict of interest
Adagmar Adriolo: no conflict of interest
José Ananias Vasconcelos Neto: no conflict of interest
João Antonio Pereira-Correia: no conflict of interest
Antonia M. O. Machado: no conflict of interest
Ana Cristina Gales: consultation fee/participation in advisory board, speaker: Abbott, Cristália, InfectoPharm, MSD, Pfizer, Zambon