To evaluate the clinical and epidemiological profile of bacterial meningitis and meningococcal disease in pediatric patients admitted to a Brazilian Secondary Public Hospital.
MethodsA descriptive observational study was conducted. Microbiologically proven bacterial meningitis or meningococcal disease diagnosed from 2008 to 2018 were included.
ResultsA total of 90 patients were diagnosed with proven bacterial meningitis. There were 64 confirmed cases of meningococcal disease. The prevalence was higher in boys (n = 38), median age 30 months (1–185). The main clinical manifestations were: meningococcal meningitis (n = 27), meningococcemia without meningitis (n = 14), association of meningococcemia with meningitis (n = 13), and fever without a known source in infants (n = 7).
Admissions to intensive care unit were necessary for 45 patients. Three deaths were notified. Serogroup C was the most prevalent (n = 32) followed by serogroup B (n = 12).
Pneumococcal meningitis was identified in 21 cases; out of the total, 10 were younger than two years. The identified serotypes were: 18C, 6B, 15A, 28, 7F, 12F, 15C, 19A and 14. Pneumococcal conjugate 10-valent vaccine covered four of the nine identified serotypes.
Haemophilus influenzae meningitis serotype IIa was identified in three patients, median age 4 months (4–7). All of them needed intensive care. No deaths were notified.
ConclusionMorbidity and mortality rates from bacterial meningitis and meningococcal disease remain high, requiring hospitalization and leading to sequelae. Our study observed a reduced incidence of bacterial disease over the last decade, possibly reflecting the impact of vaccination.
Bacterial meningitis is a serious disease and represents a substantial public health problem, with high morbidity and mortality rates.1
Mortality rates are estimated to range from 5% to 15% in all pediatric age groups. The most frequent sequelae are hearing loss, ataxia due to vestibular injury, neuropsychomotor developmental delay, paresis, epilepsy, diabetes insipidus, and blindness.2
The worldwide distribution of the disease and its epidemiology depend on factors such as etiological agent, population groups, and affected age group.3
Excluding the neonatal period, the most commonly identified bacteria in Brazil and accounting for almost 90% of the cases are: Neisseria meningitidis, Streptococcus pneumoniae and Haemophilus influenzae type b (Hib).1,4 Other less frequent but highly lethal agents may also be observed as Staphylococcus aureus (Sa).5
The recommendation of conjugate vaccines for children and adolescents in national immunization schedules has led to a notable impact on worldwide epidemiology in the last 30 years.6 Between 1990 and 2016 there was a 21% reduction in overall mortality out of 403,012 cases (95% CI: 319,426–458,514) to 318,400 (95% CI 265,218–408,705).3
The Brazilian Immunization Program offers free-of-charge vaccines that cover the three main bacterial meningitis agents.
The meningococcal C conjugate (MCC) vaccine was introduced into the National Immunization Program (NIP) in November 2010, with two doses scheduled at three and five months of age, and a booster at 12–15 months. In January 2017, the Ministry of Health included a dose of MCC vaccine for 12 to 13-year-olds, and in 2018 it was extended to 11- to 14-year-olds.7
The 10-valent pneumococcal conjugate vaccine (PCV10) was introduced in our NIP calendar in March 2010 with coverage for serotypes 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F. Doses are administered at two and four months of age, with a booster at 12–15 months.8,9
Additionally, the Hib conjugate vaccine was included in the NIP in 1999 and is scheduled at 2, 4 and 6 months of age.9
Despite reducing morbidity and mortality rates of meningitis, there is a trend for emerging new serotypes not included in the vaccines.1
To the best of our knowledge, few studies have carried out a simultaneous local analysis of these data.
Therefore, this study aimed to analyze the epidemiological and clinical evolution profile of pediatric patients admitted to a Secondary Public teaching hospital in São Paulo who were diagnosed with bacterial meningitis.
MethodsWe conducted a descriptive observational study of medical records that included pediatric patients aged from 28 days to 15 years old who were admitted at Hospital Universitário Universidade de São Paulo (HU – USP) with a suspected diagnosis of meningitis between January 2008 to December 2018.
All cases were investigated through the collection of sterile samples from blood and cerebrospinal fluid (CSF). The analysis included bacterioscopy and culture from both specimens and chemocytological analysis of CSF. Polymerase chain reaction (PCR) and/or latex agglutination testing were performed in particular cases which demanded further investigation.
All these materials were sent to the microbiological analysis laboratory of HU-USP and were processed following standardized procedures established by Institutional guidelines. Samples submitted to PCR molecular test were sent to Adolfo Lutz Institute.
Only proven bacterial meningitis or meningococcal disease were included.
Simultaneously to the collection of microbiological data aforementioned, medical records were retrieved to identify additional patients’ information including age; sex; signs and symptoms on admission such as fever, rash, vomiting, and drowsiness; major complications related to visual impairment, convulsion, hydrocephalus, septic shock, empyema, arthritis, anisocoria, and hemiparesis. Further data included length of stay, intensive care unit admission, need of vasoactive drugs support, or invasive mechanical ventilation.
Continuous variables were presented as median and interquartile range (1st to 3rd quartile) while categorical variables were presented in absolute frequencies.
This study was approved by the local Research Ethics Committee. Informed consent was waived by the Committee (CAAE: 23616819.8.0000.0076).
ResultsA total of 90 patients were diagnosed with proven bacterial meningitis.
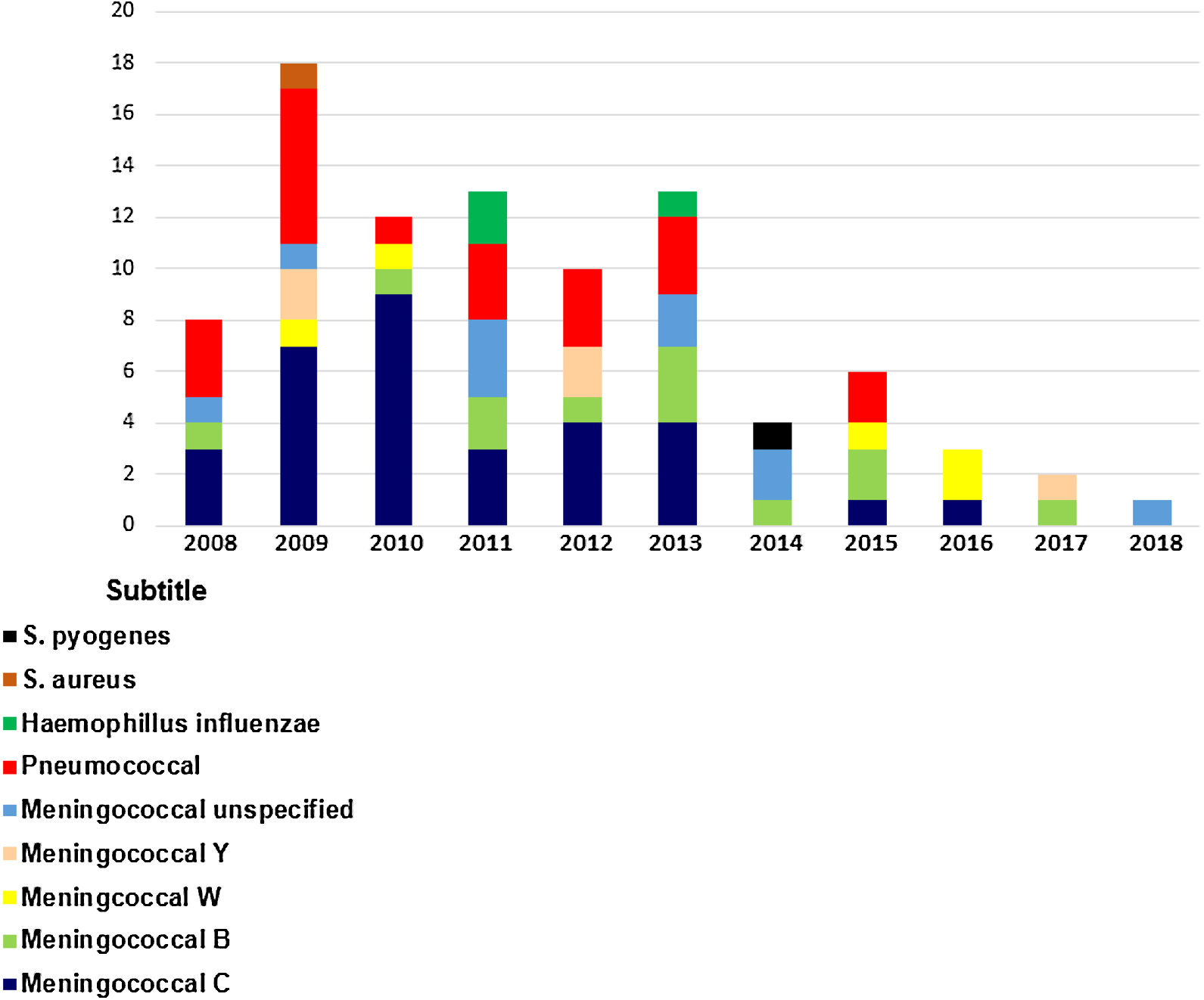
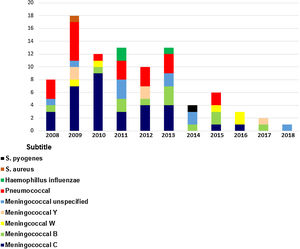
The total number of bacterial meningitis registered each year from 2008 to 2018 was 8, 18, 12, 13, 10, 13, 4, 6, 3,2 and 1, respectively (Chart 1).
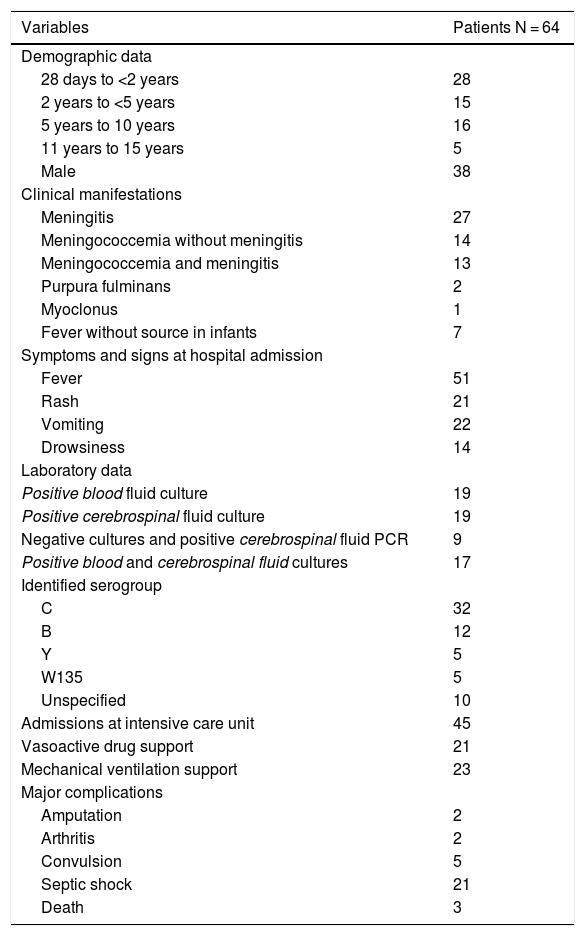
Meningococcal disease was confirmed in 64 cases during the study period. The diagnosis was more frequently observed in boys (n = 38), median age 30 months (1–185). The clinical manifestations identified were meningococcal meningitis (n = 27), meningococcemia without meningitis (n = 14), association of meningococcemia and meningitis (n = 13), purpura fulminans (n = 2), myoclonus (n = 1), and fever without a known source (n = 7).
The major complications reported were amputation (n = 2), arthritis (n = 2), convulsion (n = 5), and septic shock requiring vasoactive drug support (n = 21). Admissions to the intensive care unit were reported in 45 patients and 23 of them received mechanical ventilation support. Three deaths were notified.
As for confirmation, 17 cases were confirmed by blood and cerebrospinal fluid cultures, 19 exclusively by CSF culture, 19 exclusively by blood fluid culture, and 9 had negative cultures and positive CSF PCR. The identified serotypes were C (n = 32), B (n = 12), Y (n = 5), W135 (n = 5), and unspecified (n = 10).
In 2010, nine patients were diagnosed with meningococcal disease serogroup C (MDC) and one patient with serotype B. In 2012, there was a 55% reduction in MDC (n = 4) cases compared to 2010, and 88% reduction of MDC was (n = 1) in 2016.
From 2008 to 2013, MDC was the most prevalent serogroup notified in our hospital. However, a higher frequency of serogroup B in the last years of 2014 (n = 1), 2015 (n = 2), and 2017 (n = 2) was observed.
Table 1 shows the profile of patients diagnosed with meningococcal disease according to demographic data; clinical manifestations, symptoms and signs on admission; laboratory data, bacterial serogroups; intensive care unit admission; need for vasoactive drug support or mechanical ventilation, and major complications.
Demographic and laboratory data, clinical manifestations, symptoms and signs on hospital admission, admissions to intensive care unit, vasoactive drug support, mechanical ventilation support, and major complications of patients diagnosed with meningococcal disease.
| Variables | Patients N = 64 |
|---|---|
| Demographic data | |
| 28 days to <2 years | 28 |
| 2 years to <5 years | 15 |
| 5 years to 10 years | 16 |
| 11 years to 15 years | 5 |
| Male | 38 |
| Clinical manifestations | |
| Meningitis | 27 |
| Meningococcemia without meningitis | 14 |
| Meningococcemia and meningitis | 13 |
| Purpura fulminans | 2 |
| Myoclonus | 1 |
| Fever without source in infants | 7 |
| Symptoms and signs at hospital admission | |
| Fever | 51 |
| Rash | 21 |
| Vomiting | 22 |
| Drowsiness | 14 |
| Laboratory data | |
| Positive blood fluid culture | 19 |
| Positive cerebrospinal fluid culture | 19 |
| Negative cultures and positive cerebrospinal fluid PCR | 9 |
| Positive blood and cerebrospinal fluid cultures | 17 |
| Identified serogroup | |
| C | 32 |
| B | 12 |
| Y | 5 |
| W135 | 5 |
| Unspecified | 10 |
| Admissions at intensive care unit | 45 |
| Vasoactive drug support | 21 |
| Mechanical ventilation support | 23 |
| Major complications | |
| Amputation | 2 |
| Arthritis | 2 |
| Convulsion | 5 |
| Septic shock | 21 |
| Death | 3 |
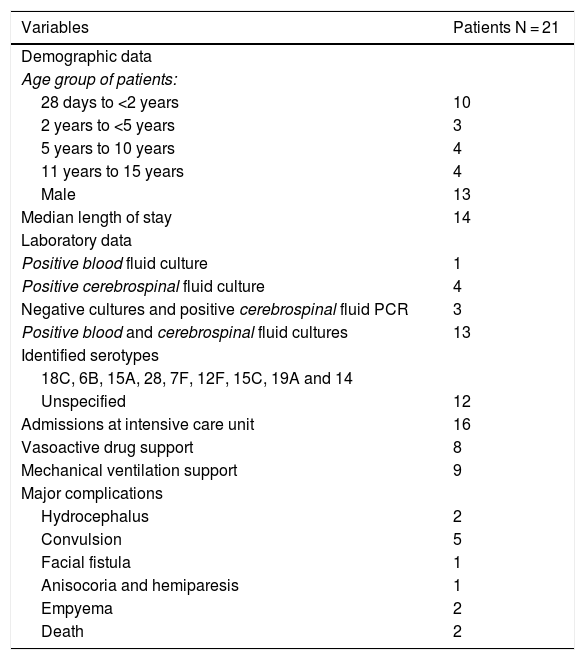
Pneumococcal meningitis was identified in 21 cases, 13 males. The age group younger than two years old accounted for 10 cases. The median length of stay was 14 days (1–65). The major complications reported were convulsion (n = 5), hydrocephalus (n = 2), facial fistula (n = 1), anisocoria and hemiparesis (n = 1), empyema (n = 2), and septic shock requiring vasoactive drug support (n = 8). Admission to the intensive care unit was reported in 16 patients and 9 of them received mechanical ventilation support. Two deaths occurred.
The last case was notified in 2015. On average, the incidence was three cases/year.
Regarding laboratory tests, 13 cases were confirmed by blood and CSF cultures, four exclusively by CSF, three had negative cultures and positive CSF PCR, and one was identified exclusively in blood culture. It was possible to identify the serotype in nine cases corresponding to 18C, 6B, 15A, 28, 7F, 12F, 15C, 19A and 14. The remaining 12 patients had no serotype identified (Table 2).
Demographic and laboratory data, median length of stay, admissions to intensive care unit, vasoactive drug support, mechanical ventilation support, and major complications of patients diagnosed with pneumococcal meningitis.
| Variables | Patients N = 21 |
|---|---|
| Demographic data | |
| Age group of patients: | |
| 28 days to <2 years | 10 |
| 2 years to <5 years | 3 |
| 5 years to 10 years | 4 |
| 11 years to 15 years | 4 |
| Male | 13 |
| Median length of stay | 14 |
| Laboratory data | |
| Positive blood fluid culture | 1 |
| Positive cerebrospinal fluid culture | 4 |
| Negative cultures and positive cerebrospinal fluid PCR | 3 |
| Positive blood and cerebrospinal fluid cultures | 13 |
| Identified serotypes | |
| 18C, 6B, 15A, 28, 7F, 12F, 15C, 19A and 14 | |
| Unspecified | 12 |
| Admissions at intensive care unit | 16 |
| Vasoactive drug support | 8 |
| Mechanical ventilation support | 9 |
| Major complications | |
| Hydrocephalus | 2 |
| Convulsion | 5 |
| Facial fistula | 1 |
| Anisocoria and hemiparesis | 1 |
| Empyema | 2 |
| Death | 2 |
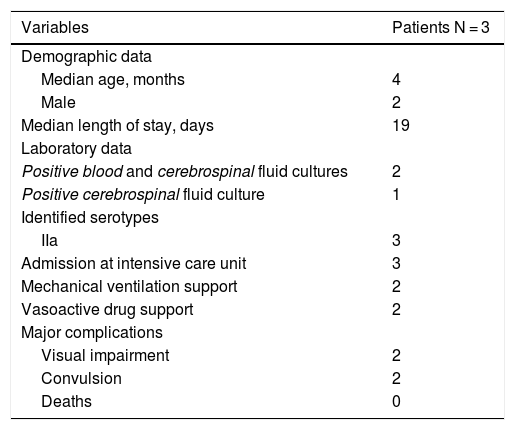
Haemophilus influenzae meningitis was identified in three patients, two of them were four-month old babies, and the remaining was a seven-month old baby. The incidence was higher in boys (2:1). The median length of stay was 19 days (14–28).
The major complications were visual impairment (n = 2), convulsion (n = 2), and septic shock requiring vasoactive drug support (n = 2). All of these patients were admitted to the intensive care unit and two of them received mechanical ventilation support. No deaths were notified.
Two cases were confirmed by blood and CSF cultures and one case exclusively by CSF culture. The identified serotype was IIa in all positive samples (Table 3).
Demographic and laboratory data, median length of stay, admissions to intensive care unit, mechanical ventilation support, vasoactive drug support, and major complications of patients diagnosed with Haemophillus influenzae meningitis.
| Variables | Patients N = 3 |
|---|---|
| Demographic data | |
| Median age, months | 4 |
| Male | 2 |
| Median length of stay, days | 19 |
| Laboratory data | |
| Positive blood and cerebrospinal fluid cultures | 2 |
| Positive cerebrospinal fluid culture | 1 |
| Identified serotypes | |
| IIa | 3 |
| Admission at intensive care unit | 3 |
| Mechanical ventilation support | 2 |
| Vasoactive drug support | 2 |
| Major complications | |
| Visual impairment | 2 |
| Convulsion | 2 |
| Deaths | 0 |
In addition, Staphylococcus aureus and Streptococcus pyogenes were isolated in two single blood cultures collected from different patients diagnosed with meningitis. They were considered true bacteremia.
The patient diagnosed with Staphylococcus aureus meningitis was a seven-year-old girl, admitted to the Emergency Department with a concomitant diagnosis of paranasal sinusitis and periorbital cellulitis. The signals and symptoms observed were fever, headache, vomiting, and ill-appearing. No mechanical ventilation or vasoactive drug support were needed. The length of stay was 15 days.
Streptococcus pyogenes meningitis was diagnosed in a four-year-old girl admitted with septic shock and purpura fulminans. The patient was transferred to the intensive care unit, requiring mechanical ventilation and vasoactive drug support. Despite appropriate empiric antimicrobial therapy, she died within 24 h of hospital admission.
DiscussionTo our knowledge, this was the first survey evaluating the local profile of bacterial meningitis in a Public Secondary Hospital in São Paulo eight years after the introduction of pneumococcal 10-valent (PCV10) and meningococcal C conjugate (MCC) vaccines into the National Immunization Program. The last study that simultaneously analyzed bacterial etiologic distribution in São Paulo State was in 20131 and no simultaneous analyses was performed in a Public Hospital in São Paulo city”.
The overall reduction of bacterial meningitis incidence through the study period is in accordance with a gradual decline incidence observed in high income countries such as Finland, Netherlands, and United States in the past two decades.6
Regarding the prevalence of meningococcal disease in this survey, serogroup C (MDC) was the most frequent in 2010, similarly to the prevalence of meningococcal disease in Sao Paulo State in the same year: 81%.10 The incidence reduction in the following years may be possibly explained by the positive impact of MCC vaccine since 2010.
National data have already suggested this vaccine impact. Between 2009 and 2010, the Brazilian invasive meningococcal disease notification rate was 7.0/100,000 for children under two years of age. In a study conducted two years after the introduction of the vaccine, a 50% decrease in the incidence was observed in vaccinated children, regardless of serogroup.8
Considering the serotype substitution, there has been a proportional increase of serotype B compared to C since 2014. Apparently, this phenomenon was not restricted to our Hospital. In 2019, the number of serotype B cases surpassed serotype C in São Paulo State (55.7% and 37.1%, respectively).11 This new trend, particularly in the age group under one-year old, stems from the proportional reduction of serogroup C cases as one of the vaccine coverage impacts.10
Regarding pneumococcal meningitis, four out of nine identified serotypes were included in the 10-valent pneumococcal conjugate vaccine (PCV10) in the post-vaccination era.
Studies have shown the effect of PCV10 on meningitis, reducing the incidence from 3.70 in 2007 to 1.84/100,000 in 2012, and the mortality rate from 1.3 to 0.4/100,000, with the greatest impact on those aged between six and 11 months.8
Another important impact is the lower colonization of nasopharynx by vaccine serotypes, which also leads to decreased transmission of these serotypes and, consequently, a reduction of invasive pneumococcal disease (IPD) in non-vaccinated groups, a phenomenon known as “herd effect”.12
On the other hand, there are also epidemiological variations between the pre- and post-vaccination period. Increased non-vaccine serotypes as a cause of IPD has been observed after 2010.13 Increase of serotype 3 is worrying because it has been associated with severe pneumonia and high mortality in adult patients.14 The serotype 19A has been associated with antimicrobial resistance.15,16 In our study, no serotype 3 was identified. One patient aged eight months died in 2015 due to pneumococcal meningitis caused by serotype 19A resistant to oxacillin and clindamycin, but sensitive to vancomycin.
Considering that less than half of our identified serotypes were included in PCV10 and given the high mortality and morbidity rate of non-vaccine pneumococcal serotypes, it is necessary to call for public health policies capable of increasing immunization serotypes coverage.
Finally, Haemophilus influenzae serotype b (Hib) infection is also highlighted as an important pathogen that, in addition to bacterial meningitis, causes other invasive diseases such as epiglottitis, pneumonia, septic arthritis, and bacteremia in children.17 Our last notified case was in 2013. Haemophilus influenzae meningitis serotype IIa was identified in three cases of bacterial disease. This low frequency may be possibly explained by the high vaccine effectiveness.
Unfortunately, there are no official national data on the incidence of Hib and non-B invasive disease before or after the introduction of the vaccine. However, data from a regional study showed a notable decline in disease incidence from 25.4 per 100,000 children with less than five years of age to 0.6 five years after immunization.17 According to records of Adolfo Lutz Institute, there has been an 88% reduction in Hib isolation strains since 1999.18
Despite high vaccination coverage, a potential increase of invasive disease caused by other serotypes has been observed in patients younger than five years.17,19 A Brazilian study analyzed 3910 cases of Haemophilus meningitis from 1990 to 2008 and concluded that identification of Haemophilus serotype non-b increased from 1% to 19% in this period while isolation of other non-Haemophilus serotypes rose from 2% to 22%.17 In our study, the serotype identified in all three patients was IIa which is consistent with the serotype substitution phenomenon.
Regarding the less common bacterial agents, the patient diagnosed with Group A Streptococcus (GAS) meningitis, was a four-year old girl, who clinically presented purpura fulminans and died two hours after admission despite vasoactive drug support and appropriate empiric antimicrobial therapy. In fact, despite uncommon in non-immunocompromised patients, GAS meningitis is associated with high mortality (17%), especially when complicated by streptococcal toxic shock.20
Of note, Staphylococcus aureus meningitis occurred in a seven-year old girl and was related to a periorbital cellulitis complication. This finding illustrates one of its pathogenic mechanisms, namely spreading from local infection.5
This study has several limitations including information risk factors and selection bias. The retrospective analysis of laboratory and medical records data missed information such as serogroups and children’s immunization status. Moreover, the total number of local bacterial disease may have been underestimated as the Pediatric Emergency Department at HU-USP has been partially closed since 2017, admitting only referred patients by other services or very critical patients. Additionally, other factors that may have influenced the epidemiology of bacterial meningitis, such as changes in sanitary conditions, were not analyzed.
In conclusion, bacterial meningitis and meningococcal disease remain associated with high morbidity and mortality rates. Our study highlights an incidence reduction of bacterial disease over the last decade and also a serotype substitution trend, possibly reflecting the impacts of immunization programs. Further longitudinal studies will be required to assess the emergence of serotypes not covered by the available vaccines. Government programs and health institutions must be able to promote public policies capable to guarantee financial, social, and human resources not only in the treatment but also in the prevention of bacterial meningitis burden in the upcoming years.
Author contributionsAll the authors contributed substantially to the conception and design of the study and in its data analysis and interpretation. Every author revised the work critically and approved the final version.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestThe authors declare no conflicts of interest.
Our gratitude to the team responsible for selecting and providing patients’ medical records according to our inclusion criteria. We also thank Adolf Lutz Institute for providing bacterial serotype identification.