Cutaneous Leishmaniasis (CL) is a noncontagious infectious disease transmitted to humans through the bites of female phlebotomines (species of mosquito) infected by protozoa of the genus Leishmania1. This insect vector belongs to the order Diptera, family Psychodidae, subfamily Phlebotominae, genus Lutzomyia. Some species of rodents, marsupials, edentates, and wild canids are known hosts.1
CL is distributed in 85 countries, with an annual record of 0.7–1.3 million new cases; it is one of the main endemic diseases of public health concern in Brazil, due to the wide occurrence of severe clinical forms and the difficulties regarding both diagnosis and treatment.2
In Brazil, this endemic condition affects the population living in the North region and is mainly related to epidemiological and environmental risk factors.3,4 In the last decades, CL has been related to the construction of large expansionist projects that have caused deforestation and migratory flows in the Northern state of Pará, thus increasing the risk areas of infection by this disease throughout the state.5
The municipality of Cametá, located in the northeastern of Pará state, has shown a significant increase in the number of CL cases, presenting evidences of relationship with environmental changes, resulting from uncontrolled urbanization, construction of highways, deforestation, among others.2,5
Considering that knowing the distribution of CL in local territorial scales can contribute to the formulation of health surveillance policies, aiming at systematic and dynamic monitoring, this study aimed to analyze the spatial distribution of CL and its relationship with epidemiological and environmental risk factors at the municipality of Cametá.
Materials and methodsThe population of this descriptive ecological study consisted of 101 reported and confirmed CL cases at municipality of Cametá, PA, from 2007 to 2016. Epidemiological data were obtained from the national Notifiable Diseases Information System (SINAN) of the municipality; demographic (population) and cartographic data (municipalities, administrative boundaries, highways, hydrography), from the Brazilian Institute of Geography and Statistics (IBGE); climatic data (precipitation), from the National Institute of Meteorology (INMET); and environmental data, from the National Institute for Space Research (INPE).
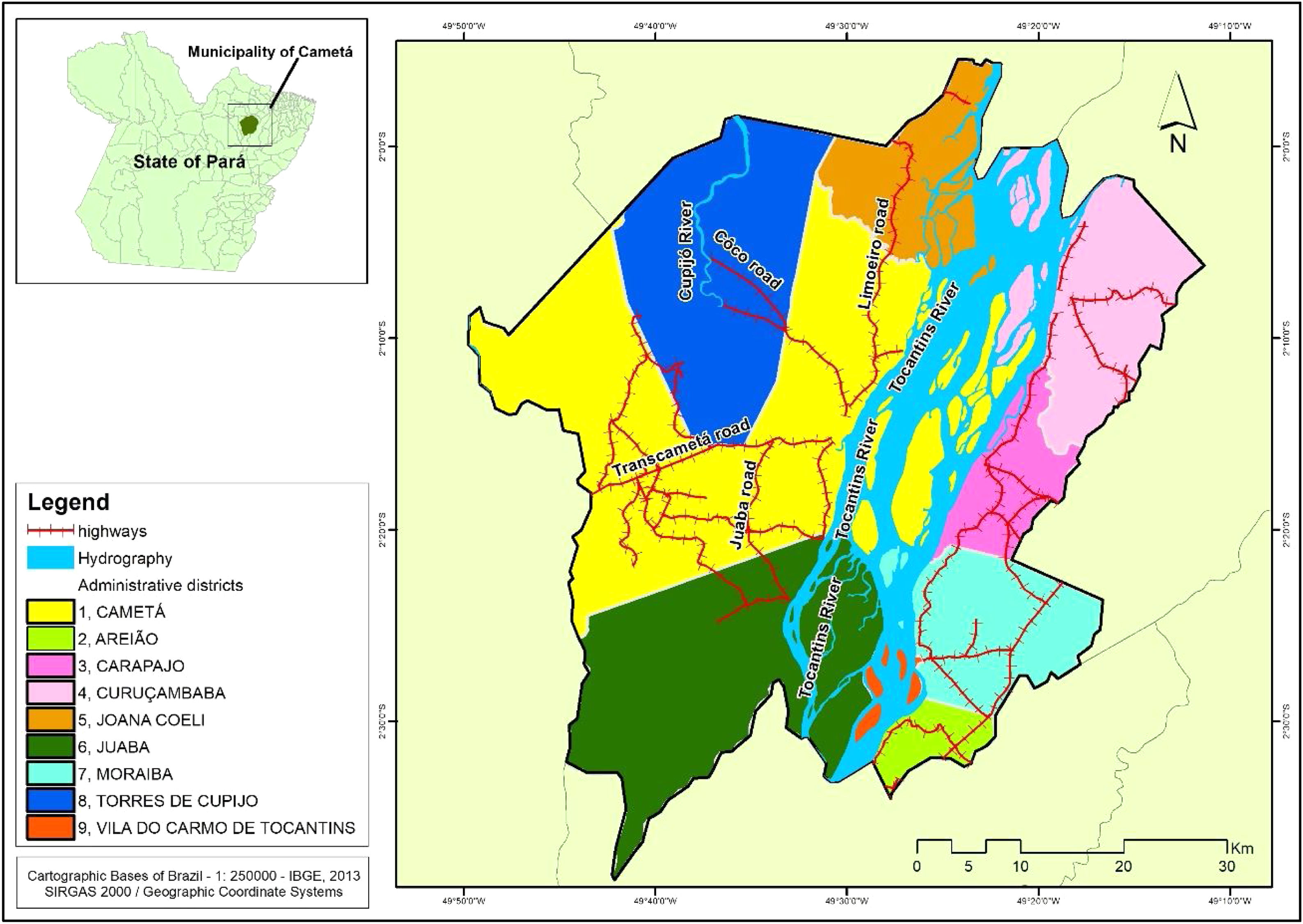
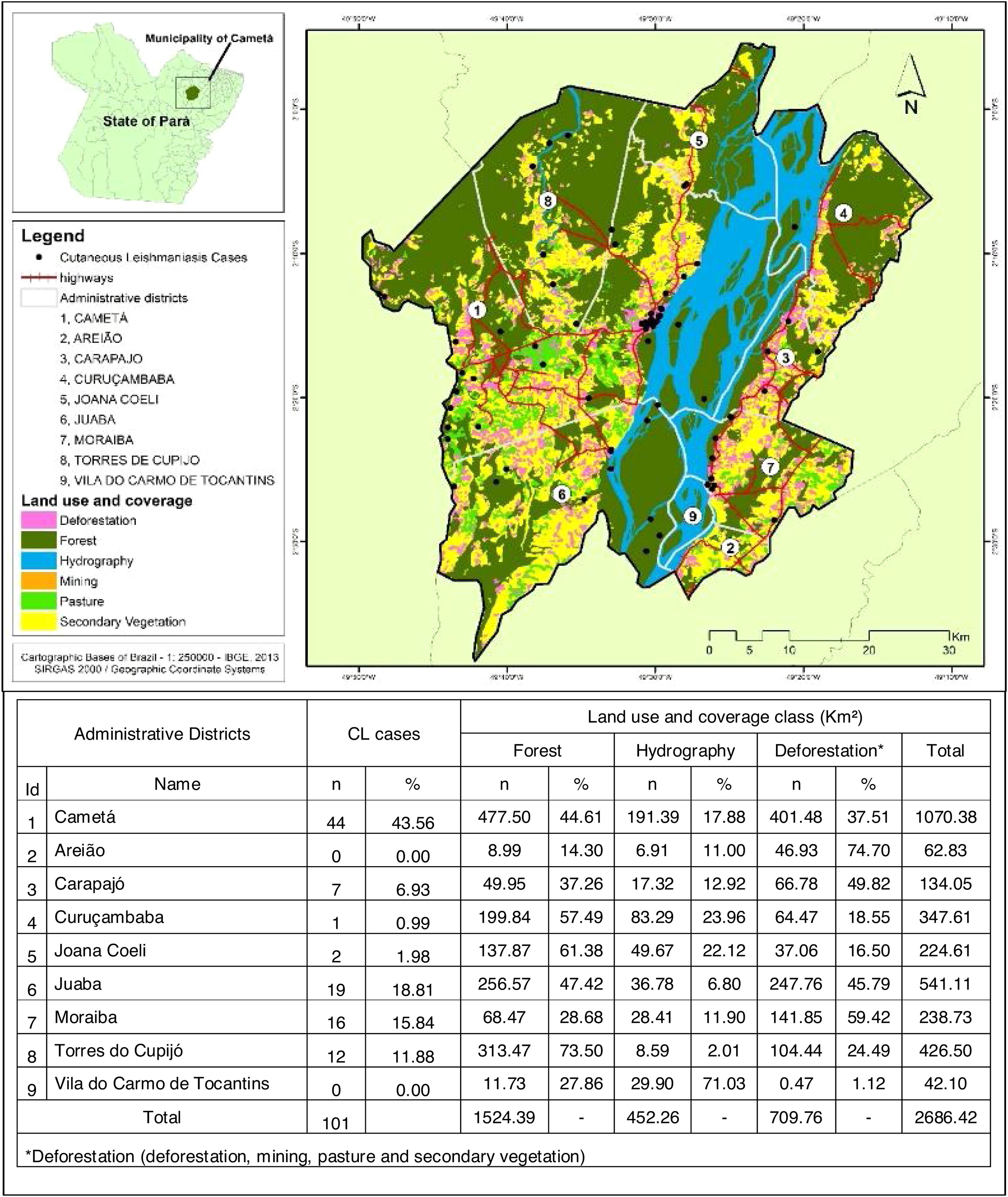
Cametá is a municipality in the state of Pará, Brazil. Located at latitude 02°14′40″ south and longitude 49°29′45″ west, altitude of 10 m, and a population of 120,896 inhabitants in 2010. It has an area of 3081 square kilometers, with a human development index (HDI) of 0.577 (low). Rainy tropical climate throughout the year, generally with temperatures ranging from 24 °C to 34 °C and temperatures below 22 °C or above 35 °C. The municipality is sub-divided into 9 districts: Cametá, Areião, Carapajó, Curuçambaba, Joana Coeli, Juaba, Moiraba, Torres do Cupijó and Vila do Carmo do Tocantins6 (Fig. 1).
After collection, database debugging was conducted to remove possible inconsistencies, using the software TabWin (http://www2.datasus.gov.br/DATASUS/index.php?area=060,805em=3). For the creation of the Geographic Database (BDGEO), data were georeferenced in the field and laboratory, using a Global Positioning System (GPS) model montana 680 and the software TerraView 4.3.3.1 (http://www.dpi.inpe.br/terralib5/wiki/doku.php?id=start). Georeferencing in urban and rural areas was carried out by collecting geographic coordinates of the house or locality where the individual with CL lived and making characterizations in the field, such as the presence of health care and basic sanitation.
In the analyses of epidemiological variables (sex, age group, race / color, education level, area, occupation), descriptive percentage calculations were performed as well as chi-square adherence test, whose p-value was <0.05, using the software Bioestat 5.3 (https://www.mamiraua.org.br/downloads/programas/). Regarding the environmental variables, the annual increase in deforestation was made available by the PRODES project (http://www.dpi.inpe.br/prodesdigital/prodesmunicipal.php), for the entire study period (2007–2016). Deforestation by area was calculated for each administrative district of the municipality, by the sum of the classes of information on use and occupation of land (deforestation, forest, hydrography, secondary vegetation, mining, and pasture) obtained from the Terraclass project (http://www.inpe.br/cra/projetos_pesquisas/dados_terraclass.php), for the year 2014, using the calculate geometry tool of the software ArcGis 10.5.
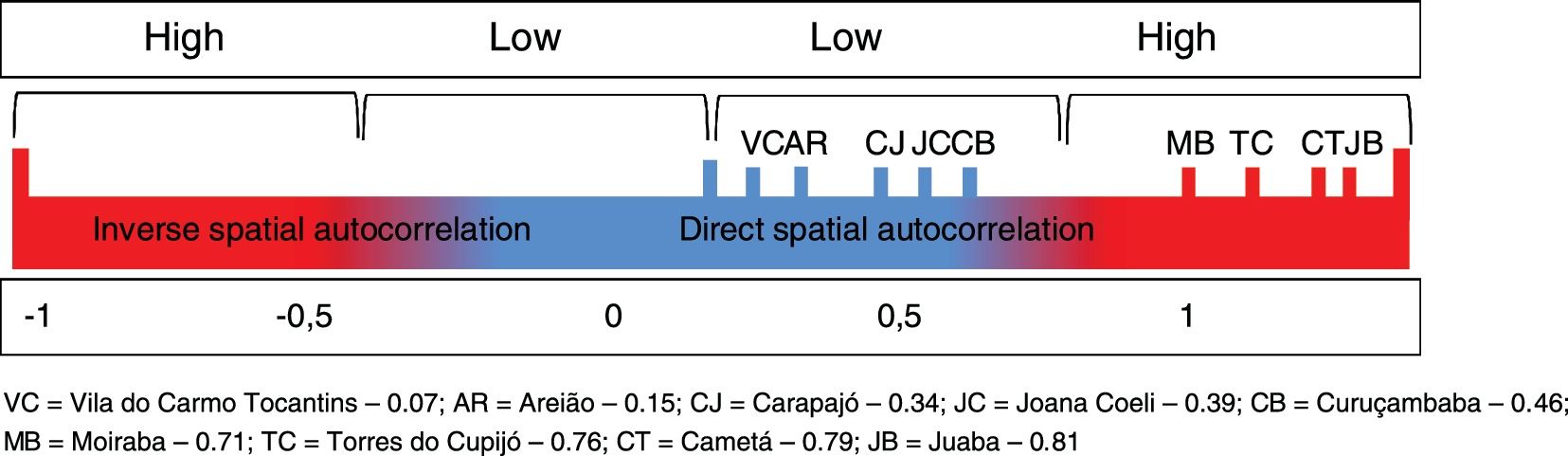
The spatial units of analysis were the administrative districts (AD) of Cametá, Areião, Carapajó, Curuçambaba, Joana Coeli, Juaba, Moraiba, Torres do Cupijó, and Vila do Carmo de Tocantins, for better representation and understanding of the disease distribution at intraregional level. Two spatial statistical techniques were used: the Local Moran's index (I), used to evaluate the spatial autocorrelation between deforestation and distribution of CL cases in the territories of the municipality. The following hypotheses of spatial autocorrelation were considered: "inverse" (I < 0); "random" (I = 0) and "direct" (I > 0). The spatial autocorrelations had two levels of intensity, strong spatial correlation for the indexes close to one of the limits [-1, 1], and weak for the indexes close to the central value [0].
The second statistical technique was the Kernel density estimator, used to evaluate the pattern of cases distribution, at a standard distance of up to 300 m between them, with the justification that values of radii lower than this interpolated only points of greater proximity, generating the density of very small areas, and, on the other hand, larger radius values generating generalized surfaces. These analyses were performed using the programs ArcGis 10.5 and TerraView 4.3.3.1.
The identity of the individuals was preserved, considering the resolution 466/2012 by National Health Council (CONEP); and opinion No. 3.245.271/2019, of the Research Ethics Committee of Universidade do Estado do Pará.
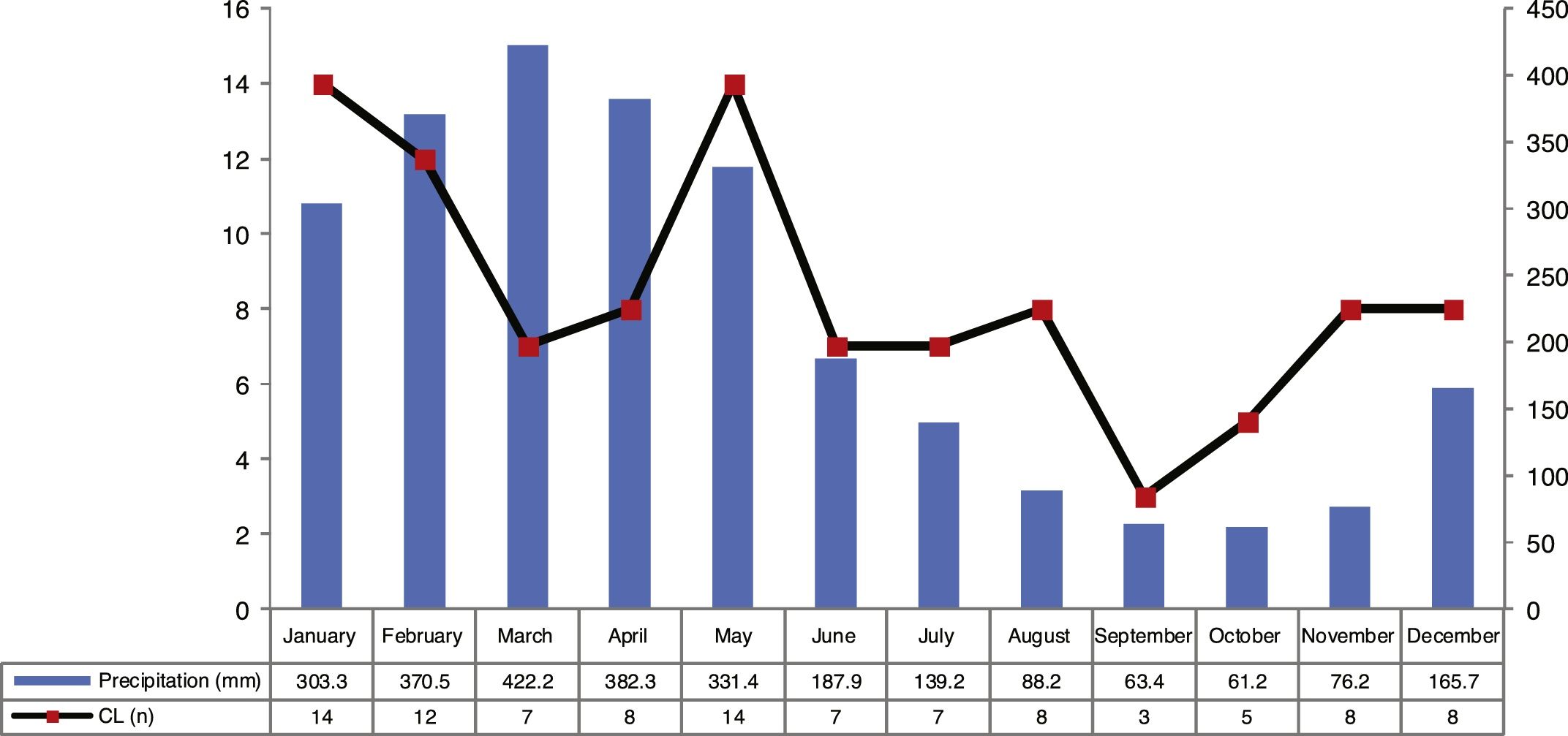
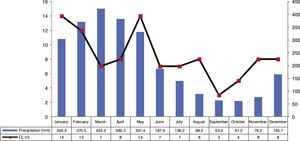
ResultsThe analysis of the cases occurring during the study period showed high prevalence of CL in municipality of Cametá with 252.35 cases per 100,000. The epidemic curve of notified CL cases and monthly precipitation levels showed a seasonal distribution pattern, with peaks or increases in the number of cases in the first semesters of the series, that is, in the rainy season. However, the number of cases decreased in the second semesters, especially in September and October, when the lowest precipitation levels were recorded, as shown in Fig. 2.
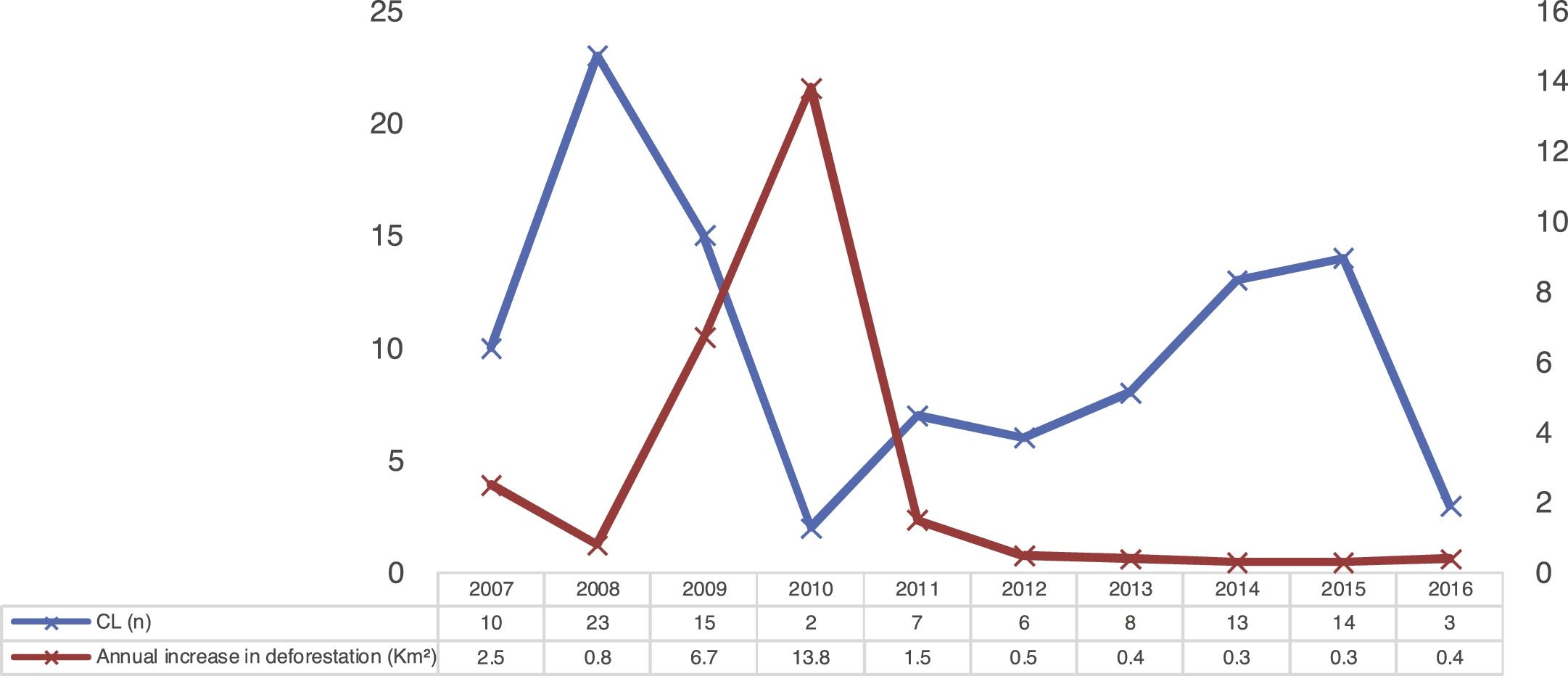
The temporal analysis did not show a pattern of direct distribution of number of CL cases with increased annual deforestation; the years with the highest number of cases presented the lowest deforestation rates, as observed in Fig. 3.
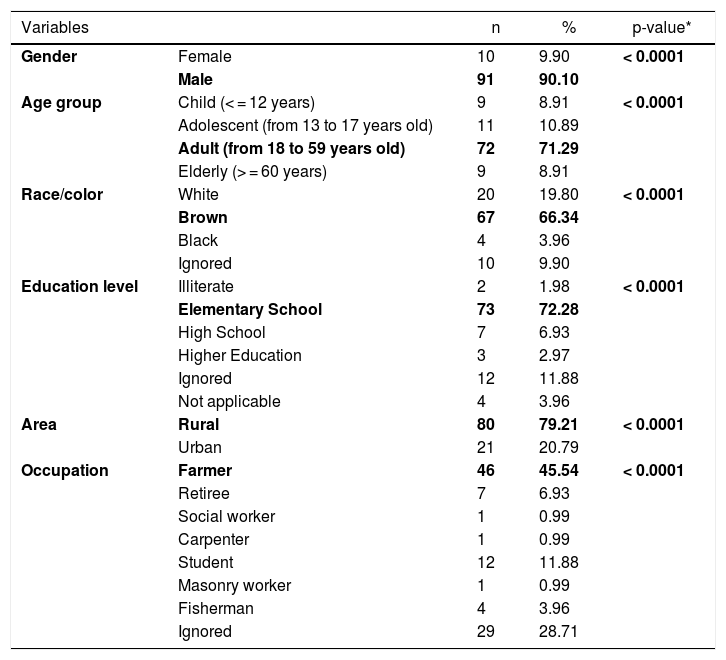
CL cases in the municipality were male (91/101), adults (72/101), of brown skin (67/101), had elementary school education (73/101), lived in rural area (80/101), presented the cutaneous form of the disease (87/101), had been discharged from the hospital for being cured (54 /101), and farmers (46/101). All epidemiological variables were significant, with p-value <0.05 (Table 1).
Epidemiological profile of cutaneous leishmaniasis, 2007 to 2016, Cametá, Pará, Brazil.
| Variables | n | % | p-value* | |
|---|---|---|---|---|
| Gender | Female | 10 | 9.90 | < 0.0001 |
| Male | 91 | 90.10 | ||
| Age group | Child (< = 12 years) | 9 | 8.91 | < 0.0001 |
| Adolescent (from 13 to 17 years old) | 11 | 10.89 | ||
| Adult (from 18 to 59 years old) | 72 | 71.29 | ||
| Elderly (> = 60 years) | 9 | 8.91 | ||
| Race/color | White | 20 | 19.80 | < 0.0001 |
| Brown | 67 | 66.34 | ||
| Black | 4 | 3.96 | ||
| Ignored | 10 | 9.90 | ||
| Education level | Illiterate | 2 | 1.98 | < 0.0001 |
| Elementary School | 73 | 72.28 | ||
| High School | 7 | 6.93 | ||
| Higher Education | 3 | 2.97 | ||
| Ignored | 12 | 11.88 | ||
| Not applicable | 4 | 3.96 | ||
| Area | Rural | 80 | 79.21 | < 0.0001 |
| Urban | 21 | 20.79 | ||
| Occupation | Farmer | 46 | 45.54 | < 0.0001 |
| Retiree | 7 | 6.93 | ||
| Social worker | 1 | 0.99 | ||
| Carpenter | 1 | 0.99 | ||
| Student | 12 | 11.88 | ||
| Masonry worker | 1 | 0.99 | ||
| Fisherman | 4 | 3.96 | ||
| Ignored | 29 | 28.71 | ||
Source: Research protocol, 2019.
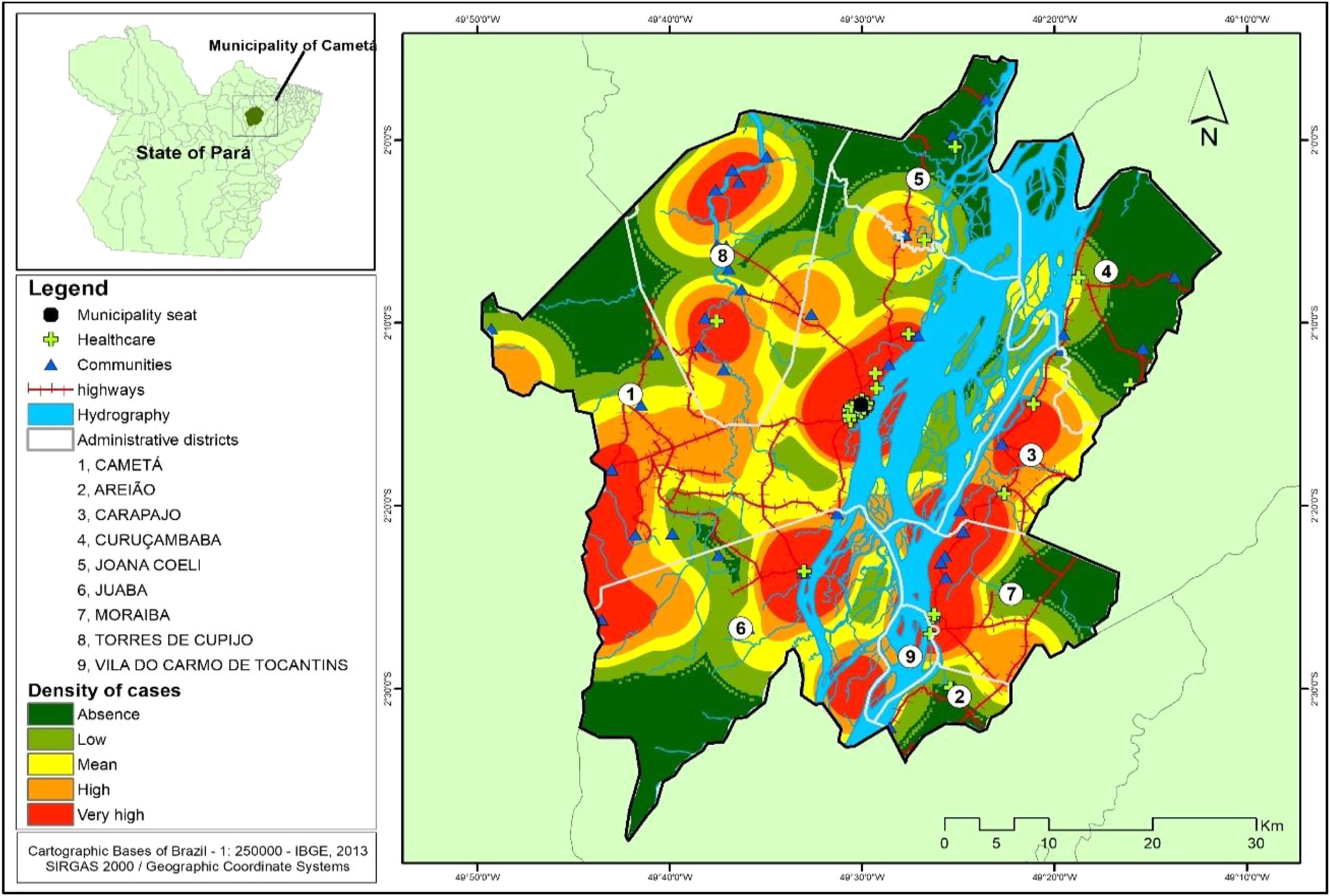
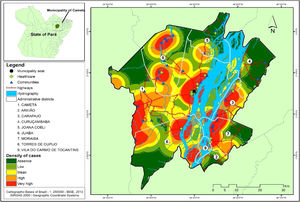
Several clusters of cases were observed, considering the space in which they were located. This procedure showed the highest concentration in urban area, even though it is not the area with the largest number of cases (21). In turn, the rural area (80), where the largest number of cases was identified in a more dispersed way in the geographic space, had several places with high density of cases, as shown in Fig. 4.
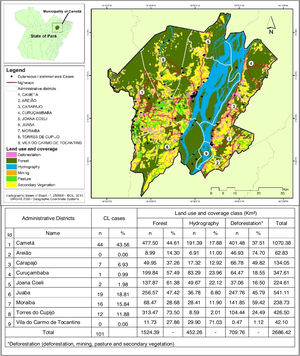
Regarding the distribution of CL cases related to land use and coverage, the districts where the cases occurred were those with the highest percentage of accumulated deforestation. The highest percentage of cases was observed in AD of the Cametá (43.56%), as well as a high percentage of deforestation (37.51%), followed by the ADs of Juaba, with 18% and 45.79%; Moraiba, with 15.84% and 59.42%; Torres do Cupijó, with 11.88% and 24.49%; Carapajó, with 6.93% and 49.82%; Joana Coeli, with 1.98% and 16.50%; and Curuçambaba, with 0.99% and 18.55%, respectively. The districts of Areião and Vila do Carmo de Tocantins did not notify cases, but Areião was the AD with the highest percentage of deforested area, with 74.70%, as shown in Fig. 5.
Local Moran’s Index (I) showed significant spatial relation between deforested areas and location of CL cases in the administrative districts of Cametá. Thus, a direct autocorrelation between these two variables was observed, and positive (I > 0) and significant (p < 0.0001) indexes were found in these areas. The spatial autocorrelations were strong in the districts of Cametá (0.79), Juaba (0.81), Moraiba (0.71), and Torres do Cupijó (0.76), as shown in Fig. 6.
DiscussionDuring the study period, Cametá had the highest prevalence of CL in the northeast of Pará. This finding may be associated with the particularities of the demographic dynamics, the precarious housing conditions of the population, the migratory flow, especially in the ADs of Moraiba and Torres do Cupijó, where forests (natural ecotopes of phlebotomines) as well as economic activities of livestock, hunting, and family farming are found.7,8,15
In the Cametá and Juaba AD, the presence of pets was observed, hematophagous insects, rodents, an uncontrolled expansion of the urban area, where the population remained very close to the forest, influencing the permanence of the disease in peridomiciles (artificial ecotopes of phlebotomines).9
The seasonal distribution of notified and confirmed CL cases was more significant in the first semesters of the years analyzed, especially in January and May, comprising the rainy seasons of the year, in which precipitation indices are higher. The hot and humid climate of the region is a breeding ground for the mosquito.10,11
There is an important relationship between the use of the soil and the location of the disease, besides the proximity of the houses to the forest. Thus, increase in case numbers and of the temperature in the first semesters may be related to the dispersion of the vectors of the disease, which go from their wild environment to the human peridomicile, increasing the chances of vector transmission.12,13
Considering that the epidemiological profile showed that most cases in the cities affected males, adults, with low schooling, residents in rural areas, and farmers,3,5,13 the relation with some risk factors for transmission of the disease is possible. One example is the population’s lack of knowledge or disuse of individual and/or collective protection against the attack by phlebotomines. On the part of the managers, there is poor understanding of the dynamics of transmission of the disease and a low availability of health units in the rural areas of the municipality.14
The spatial distribution of CL cases in the territory studied showed the disease behaves in a nonhomogeneous way5,14 following a tendency possibly related to the process of environmental degradation along the country roads. For example, at roads of Côco, Transcametá, Limoeiro, and Juaba, located in west of the municipality, and on the banks of the rivers Tocantins and Cupijó, due to the strong trade with intense economic activity, linked to wood and coal. Moreover, regarding the extraction of non-timber products, over the last decades floodplain areas have recently undergone an economic revitalization due to increased management of açaí into areas where conditions are prone to disease transmission.
In the municipality of Cametá, districts with high levels of accumulated deforestation also notified the largest number of CL cases. Thus, the relation between CL and deforestation in these areas showed strong evidence of segmentation in the territories studied, mainly in urbanized areas12,14 and on the banks of the rivers Tocantins and Cupijó.
The spatial autocorrelations between the distribution of CL and accumulated deforestation may be related to old areas of deforestation that are established there and the type of activity developed by the local population. Along the roads, the main activity is family farming; at the riverbanks near areas, livestock is the main activity. These facts were previously observed in the field visit.15,16
ConclusionA large number of CL notifications were observed in the municipality of Cametá from 2007 to 2016, with a high rate of disease in the first semesters, the rainiest period. It shows that the scenario facilitates vector development, associated with a double epidemiological burden, both for agricultural activities in expansion in these historically deforested areas, and for the endemic characteristics of the municipality, with persistent outbreaks of cases for transmissibility of the disease.
Consistently adopting the long-term measures of vector control (indoor residual spraying and destruction of breeding spots), health education, active search for cases and diagnosis, as well as early treatment are recommended. Regarding the environmental issue, we emphasize the need for intensifying surveillance actions throughout the municipality territory, mainly near the banks of the river Cupijó. A more effective monitoring of the uncontrolled demographic expansion in susceptible areas to the disease is also important.
ContributionsThe authors contributed equally
Conflict of interestThe authors declare no potential conflict of interests.
To the Laboratory of Epidemiology and Geoprocessing of Amazon (EPIGEO), from Universidade do Estado do Pará (UEPA); To Universidade Federal Rural da Amazônia (UFRA); To the Cametá Department of Health and to the Brazilian National Research Council (CNPq).