Differential diagnosis of COVID-19 includes a broad range of conditions. Prioritizing containment efforts, protective personal equipment and testing can be challenging. Our aim was to develop a tool to identify patients with higher probability of COVID-19 diagnosis at admission.
MethodsThis cross-sectional study analyzed data from 100 patients admitted with suspected COVID-19. Predictive models of COVID-19 diagnosis were performed based on radiology, clinical and laboratory findings; bootstrapping was performed in order to account for overfitting.
ResultsA total of 29% of patients tested positive for SARS-CoV-2. Variables associated with COVID-19 diagnosis in multivariate analysis were leukocyte count ≤7.7×103mm–3, LDH >273U/L, and chest radiographic abnormality. A predictive score was built for COVID-19 diagnosis, with an area under ROC curve of 0.847 (95% CI 0.77–0.92), 96% sensitivity and 73.5% specificity. After bootstrapping, the corrected AUC for this model was 0.827 (95% CI 0.75–0.90).
ConclusionsConsidering unavailability of RT-PCR at some centers, as well as its questionable early sensitivity, other tools might be used in order to identify patients who should be prioritized for testing, re-testing and admission to isolated wards. We propose a predictive score that can be easily applied in clinical practice. This score is yet to be validated in larger populations.
As a result of its broad clinical presentation – from asymptomatic infection to severe acute respiratory syndrome (SARS) – as well as non-specific laboratory and radiological features, differential diagnosis of COVID-19, particularly at admission, is challenging. Influenza, pneumonia, tuberculosis, arbovirus infections, and even non-infectious illnesses have been reported as conditions that can mimic COVID-19.1–3
Identification of patients at high-risk for COVID-19 before confirmatory testing is key to prioritize containment efforts, RT-PCR tests, and personal protective equipment (PPE), especially in hospital settings. Considering that RT-PCR sensitivity is variable – in some reports, it is as low as 71%,4 patients at high-risk for COVID-19 with a negative RT-PCR result should be re-tested before being transferred to a non-COVID ward. However, there is little knowledge on how to identify patients who have a higher pre-test probability for COVID-19.
In this study we analyzed significant variables associated with initial COVID-19 diagnosis. Our objective was to develop a useful predictive tool for COVID-19 diagnosis based on clinical, laboratory and image data prior to RT-PCR test confirmation. ROC Curve models and a clinical score are provided aiming to predict early SARS-CoV-2 infection diagnosis.
MethodsThe first 118 consecutive patients aged 18 or older admitted to Hospital de Clínicas de Porto Alegre, an 831 bed tertiary referral hospital located in Southern Brazil, due to suspected COVID-19 were assessed. Clinical, laboratory and radiological findings at presentation were analyzed. Patients discharged within 24h of admission were excluded. This study protocol was approved by the institutional review board, and informed consent was obtained from each patient or closest family member.
Upon admission, samples of nasal and throat swabs were collected by trained healthcare professionals according to the American Centers for Disease Control and Prevention (CDC) guidelines. The sample was used for real-time polymerase chain reaction (RT- PCR), designed to detect three regions of the virus nucleocapsid (N1, N2, N3), according to the CDC diagnostic panel.5 Patients undertook chest image exams before RT-PCR results; therefore, radiologists were unaware of the patient diagnosis.
Patients were divided into those who had positive RT-PCR for SARS-CoV-2 and those with negative results. Kruskal–Wallis test, χ2 test and Fisher's exact test were used to compare differences between groups.
For our predictive score, variables associated with outcome (defined as p≤0.05) in univariate analysis were tested in a logistic regression model. Each significant variable received points based on multivariate model odds ratio (OR). Variables that showed no significant association with COVID-19 diagnosis in univariate analysis were not included. The final model included abnormality on chest radiography, LDH levels, and leukocyte count. Bootstrapping was performed to assess overfitting of the models. Development of this score was performed according to TRIPOD guidelines.6
ResultsPatients were admitted from March 17 to April 10, 2020; 18 were excluded because they had been discharged within 24h of admission. Out of 100 patients, 29 were SARS-CoV-2 positive. Main characteristics are listed in Table 1.
Clinical, laboratory and radiographic characteristics of patients with suspected SARS-CoV-2 infection at admission.
| All (n=100) | Confirmed (n=29) | Negative (n=71) | p-Value | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age | 58 (40–69.5) | 62 (56–69) | 54 (34–68) | 0.06 |
| Male | 43 (43%) | 15 (51.7%) | 28 (39.4%) | 0.26 |
| Fever | 67 (67.7%) | 27 (93.1%) | 40 (57.1%) | <0.001 |
| Dyspnea | 65 (65.6%) | 20 (68.9%) | 45 (64.2%) | 0.65 |
| Cough | 69 (69%) | 21 (72.4%) | 48 (68.5%) | 0.70 |
| Expectoration | 20 (20%) | 3 (10.3%) | 17 (24.3%) | 0.1 |
| Chest pain | 23 (23%) | 3 (10%) | 20 (28.6%) | 0.05 |
| Headache | 34 (34%) | 13 (44.8%) | 21 (30%) | 0.15 |
| Myalgias | 44 (44%) | 16 (55.2%) | 28 (40%) | 0.17 |
| Asthenia | 40 (40%) | 15 (51.7%) | 25 (35.7%) | 0.14 |
| URT Symptoms | 37 (37%) | 10 (34.5%) | 27 (38.6%) | 0.7 |
| GI Symptoms | 38 (38%) | 13 (44.8%) | 25 (35.7%) | 0.72 |
| Respiratory distress | 18 (18%) | 2 (6.9%) | 16 (22.5%) | 0.06 |
| PSI/PORT score | 0.007a | |||
| ≤70 | 38 (45.7%) | 16 (57.1%) | 22 (40%) | |
| 71–90 | 13 (15.6%) | 8 (28.6%) | 5 (9.1%) | |
| 91–30 | 18 (21.7%) | 2 (7.1%) | 16 (29.1%) | |
| >130 | 14 (16.8%) | 2 (7.1%) | 12 (21.1%) | |
| Comorbidities | ||||
| Hypertension | 40 (40%) | 10 (34.5%) | 30 (42.2%) | 0.5 |
| Ever smoker | 36 (36%) | 8 (27.6%) | 28 (40%) | 0.24 |
| Lung disease | 30 (30%) | 5 (17.2%) | 25 (35.2%) | 0.07 |
| Heart disease | 18 (18%) | 2 (6.9%) | 16 (22.5%) | 0.05 |
| Diabetes | 18 (18%) | 3 (10.7%) | 15 (21.1%) | 0.2 |
| Obesity | 12 (12%) | 8 (11.3%) | 4 (14.2%) | 0.7 |
| Malignancy | 11 (11%) | 1 (3.4%) | 10 (14%) | 0.1 |
| Number of comorbidities | 0.005a | |||
| No comorbidities | 32 (33%) | 14 (51.8%) | 18 (25.7%) | |
| 1–2 | 48 (49.5%) | 13 (48.2%) | 35 (50%) | |
| ≥3 | 17 (17.5%) | 0 | 17 (24.2%) | |
| Laboratory findings | ||||
| Hemoglobin, g/dL | 12.7 (11.1–13.8) | 13.2 (12.6–14.3) | 12.2 (10.5–13.7) | 0.06 |
| RDW (%) | 13.2 (12.4–14.5) | 12.8 (12.1–13.2) | 13.5 (12.6–14.9) | 0.006 |
| Platelet count, ×103 per mm3 | 207 (170–275) | 194 (175–248) | 212 (170–278) | 0.54 |
| Leukocyte count, ×103 per mm3 | 9.9 (6.3–13.3) | 6.4 (5.3–9.9) | 11.7 (8.1–15.4) | <0.001 |
| <7.7 | 32 (32%) | 19 (65.5%) | 13 (18.3%) | <0.001 |
| Lymphocyte count, ×103 per mm3 | 1.2 (6.5–1.8) | 0.9 (0.6–1.3) | 1.3 (6.5–2.1) | 0.04 |
| <1 | 42 (42%) | 15 (51.7%) | 27 (38%) | 0.2 |
| Neutrophil/lymphocyte ratio | 6.25 (3.1–12.2) | 5.14 (3.1–7.7) | 6.9 (3.5–13.4) | 0.3 |
| <3.13 | 23 (23%) | 7 (24.1%) | 16 (22.5%) | 0.8 |
| Creatinine, mg/dL | 0.97 (0.76–1.28) | 0.99 (0.77–1.14) | 0.96 (0.76–1.34) | 0.13 |
| ≥1.33 | 24 (24%) | 4 (13.8%) | 20 (28.2%) | 0.12 |
| Urea, mg/dL | 32 (23–51.5) | 29 (23–42) | 36 (23–58) | 0.1 |
| Lactate dehydrogenase, U/L | 256 (186–379) | 344 (258–421) | 213 (182–297) | 0.004 |
| ≥273 | 34 (45.3%) | 18 (72%) | 16 (32%) | 0.001 |
| Creatine kinase, U/L | 77.5 (50–129) | 93.5 (49–139) | 74 (50–119) | 0.17 |
| >185 | 8 (11.4%) | 4 (18.8%) | 4 (8.3%) | 0.23 |
| D-dimer, μg/L | 1.17 (0.46–2.17) | 1.29 (0.58–1.73) | 1.14 (0.37–2.25) | 0.59 |
| ≤0.5 | 24 (30%) | 4 (16.7%) | 20 (35.7%) | 0.08a |
| >0.5 | 56 (70%) | 20 (83.3%) | 36 (64.3%) | |
| C-Reactive Protein, mg/dL | 73.1 (19.2–152.9) | 87 (47–142) | 58.1 (14.7–154) | 0.3 |
| >100 | 37 (38.5%) | 12 (42.8%) | 25 (36.7%) | 0.6 |
| Serum lactate levels, mmol/L | 1.3 (1–2.2) | 1.1 (0.9–1.3) | 1.64 (1–2.3) | 0.009 |
| ALT, U/L | 24 (15–45.5) | 34.5 (23–56) | 19 (12–36) | 0.018 |
| >40 | 22 (28.9%) | 10 (45.4%) | 12 (22.2%) | 0.04 |
| INR | 1.13 (1.07–1.25) | 1.08 (1.03–1.17) | 1.17 (1.08–1.3) | 0.02 |
| ≤1.2 | 48 (66.7%) | 17 (80.9%) | 31 (60.8%) | 0.09a |
| >1.2 | 24 (33%) | 4 (19%) | 20 (39.2%) | |
| Radiographic findings | ||||
| Consolidation | 34 (34%) | 15 (51.7%) | 19 (27.1%) | 0.02 |
| Infiltration | 33 (33%) | 13 (44.8%) | 20 (28.6%) | 0.12 |
| Ground-glass opacity | 26 (26%) | 19 (65.6%) | 7 (10%) | <0.001 |
| Pleural effusion | 6 (6%) | 1 (3.4%) | 5 (7.1%) | 0.48 |
| Lower lobe predominance | 60 (61%) | 25 (86.2%) | 35 (50.7%) | 0.001 |
| Bilateral involvement | 47 (67.1%) | 22 (75.9%) | 25 (60.9%) | 0.19 |
| Normal imaging | 30 (30%) | 1 (3.4%) | 29 (41.4%) | <0.001 |
Data presented as Median (IQR), n (%). p-Values calculated using χ2 test, Fisher's exact test, or Kruskal–Wallis test.
Four patients (13.7%) of the confirmed group were hospital healthcare staff. In the negative RT-PCR group, six (21.4%) patients had no contact with confirmed or suspected cases, compared to 58 (82.8%) in the confirmed group (p<0.001). No patients in the negative group had traveled in the previous three weeks, compared to eight (28.6%) patients in the confirmed group (p<0.001).
Patients in the negative group were more likely to have a higher number of comorbidities and to present with a higher PSI/PORT index. There was no difference in CURB-65 score among groups (p=0.10).
The optimal cut-off value was 273U/L for LDH and 7.7×103 per mm3 for leukocyte count. Results from the multivariable analyses are presented in Table 2.
Of the 29 patients who tested positive for SARS-CoV-2, four had a previous negative result. For the remaining 71 who were considered negative, 19 (26%) were re-tested – 15 of them because the first test was inconclusive, and four patients because of clinical suspicion.
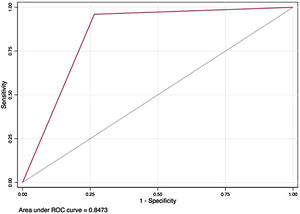
Predictive scoreA predictive score for COVID-19 was built including variables that had shown an association with outcome in multivariate analysis; it yielded two points for LDH >273U/L, three for leukocyte count ≤7.7×103 per mm3 and four for any chest radiography abnormality. A result ≥5 points was considered positive. This score had 96% sensitivity and 73.5% specificity in our sample, with an area under ROC curve (AUC) of 0.847 (95% CI 0.77–0.92), as seen in Fig. 1.
After bootstrapping, this model had an AUC of 0.827 (95% CI 0.75–0.90) (Fig. 1).
DiscussionRT-PCR TESTINGTwo patients in the COVID-19 group had tested negative at admission; after negative results, they were transferred to non-COVID wards. Because of clinical suspicion, these patients were re-tested, yielding positive results.
Admitting a COVID-19 patient into a regular ward can be catastrophic. We advocate that patients who are to be moved to regular wards be re-tested before they are transferred; if testing all of them was not possible, patients with higher probability for COVID-19 – using a combination of clinical, laboratory and radiological data – should be prioritized for testing. Those who are discharged with one negative RT-PCR result should be advised to remain isolated at home for two weeks.
In addition, two other cases in the positive group were health workers who had tested negative two days before retesting. Infected health care workers are likely to transmit SARS-CoV-2 to patients with comorbidities – which are at higher risk for severe infection. Hence, we highlight the need for more than one negative result in order to allow health care workers to return to work. Although the CDC advocates the need for at least two negative nasopharyngeal swab specimens,7 some local guidelines around the world require only one negative test.8
Predictive scoreThere is increasing need for a validated clinical score estimating COVID-19 probability. Considering shortage of PPE, PCR testing and isolation units in many countries around the world, as well as non-ideal sensitivity of nasopharyngeal swabs, doctors at Emergency and Intensive Care Units should be provided with clinical tools, other than contact history, in order to prioritize isolation and testing. A combination of clinical findings, laboratory data and radiological patterns (when available) can be useful in this context.
Our predictive score yielded good sensitivity and specificity after adjusting for overfitting. Fever was not included in the model because patients without fever are rarely admitted for suspected COVID-19.
Clinical presentationMost patients with confirmed COVID-19 diagnosis had fever, consistent with data published so far on clinical manifestations of COVID-19.9–11 Most of them had traveled and/or had contact with a suspected case; this was expected, as our study sample reflects some of the first cases of COVID-19 in our region and most of them were infected before community transmission was declared.
In our sample, patients without COVID-19 were more likely to present with a higher PSI/PORT at admission. We believe these higher scores reflect a higher number of comorbidities and increased severity in the differential diagnosis group, which could explain their increased serum lactate and bilirubin levels at admission. In addition, patients who were returning travelers, and those who had a positive contact history for SARS-CoV-2 seemed to be more prone to have an early hospital presentation. We have excluded patients who were discharged within 24h to diminish this bias.
Our analysis did not have enough power to demonstrate a significant association for each of the included comorbidities; however, there was an inverse association between number of comorbidities and COVID-19 diagnosis. This can be explained by a number reasons: patients with other comorbidities are more likely to present with severe bacterial pneumonia and sepsis, for example; also, almost a third of COVID-19 cases had traveled in the previous three weeks, compared to none in the other group. Patients with more comorbidities might be less able to travel – which might have lessened the probability of being infected in the first days of transmission in Brazilian territory. As community transmission increases in our region, we believe the contrast in number of comorbidities between COVID-19 and other respiratory conditions may decrease.
Laboratorial findingsMany studies have found that COVID-19 correlates with lower leukocyte count.12–14,16 Relationship between this finding and severity of COVID-19 cases is unknown; some studies have reported that more severe cases had lower leukocyte count12 while some have reported the inverse.9 In our cohort, a lower leukocyte count (however not sufficient to be categorized as leukopenia) was a predictor for COVID-19 diagnosis. This might be due to marked leukocytosis of some differentials, for instance bacterial infections.
In our sample LDH was a strong predictor for COVID-19 diagnosis. LDH is a predictor of inflammation in several lung diseases. Consistently with data from previous studies,10 higher LDH levels were associated with COVID-19 diagnosis. Most studies revealed that it is a good predictor of severity and ICU admission.10,12,15
Elevation of ALT and AST have already been described as severity markers; in our study higher levels of ALT were associated with COVID-19 diagnosis. In a systematic review of COVID-19 presentation, 17.2 to 28.3% had elevated ALT, and 29.4 to 75.8% had elevated LDH.17
In our sample, CRP and D-dimer levels were markers of disease severity and associated with ICU admission and higher CURB-65 at admission, irrespective of COVID-19 diagnosis. In a study by Guan et al., CRP was elevated in 60% of COVID-19 patients upon arrival, also associated with ICU admission12; some articles reported an association between CRP levels and extension of pulmonary involvement.15
The lack of a significant association between COVID-19 diagnosis and CRP levels in this study may be due to a small sample size and also increased severity of presentation for non-COVID patients. Also, we have only used CRP levels upon admission; perhaps CRP increase would occur later on in COVID-19 presentation.
Image findingsIn our patients submitted to chest CT, the most relevant image findings were ground-glass opacities. In addition, positive cases usually presented with lower lobe predominance; this is consistent with some of the data published so far on radiological patterns of COVID-19,17 although some studies have not found a lobe predominance.18
It is worth to highlight that in our sample, only one COVID-19 patient had normal chest image. Radiographic patterns associated with COVID-19 are unspecific, indicating the need to correlate imaging with clinical data.
Contact historyEven though report of contact with patients who were positive for SARS-CoV-2 increased accuracy of our score, it was not included as a variable. Once local transmission is established within a region, tracking an epidemiological history becomes impractical.
Differential diagnosesAlthough Influenza is reported as one of the most important differential diagnoses of COVID- 19, we had no cases reported, probably due to seasonality; in our region, Influenza cases peak by June.19 In winter months, we also expect higher rates of COPD exacerbation; differentiating between these conditions will be challenging.
Excluding COVID-19 is burdensome in a number of ways, but especially in the emergency department, considering COVID-19 can be asymptomatic.20 One of our patients sought medical assistance for acute onset of hemiparesis and tested positive for SARS-CoV-2 after a head and neck computed tomography angiography with an incidental finding of ground glass opacities in both superior lung lobes. Among a group of pregnant women admitted for delivery in a New York City hospital, Sutton et al.21 found 13% of asymptomatic patients tested positive. Due to lack of resources, universal testing is not a plausible option, therefore high clinical suspicion, along with low threshold for chest imaging, is advisable.
LimitationsOur study has some limitations, aside from intrinsic limitations of an observational study; most of them are associated with data collected from medical records. Visiting and interviewing patients was not possible at all times; collecting data from patients in mechanical ventilation was challenging and likely to be compromised since it was not always possible to reach for family members.
Our control population has a high number of comorbidities and may not represent the general public; in addition, optimal cut-points for this study may not be the same in other samples. Our study has a small sample size and may not have enough power to evaluate other variables that might be associated with COVID-19 diagnosis. It is important to note that this score has not yet been validated.
Another limitation of our study was the unavailability of a second negative PCR testing for the ruled-out group. Of 23 patients who were re-tested, four turned out positive at re-testing; it is possible that some patients classified as negative would result positive in further testing, particularly those with inconclusive diagnosis. This can also underestimate relationships between COVID- 19 diagnosis and some of the variables analyzed.
Nevertheless, considering the need to segregate in a special ward a patient with a low pre- test probability of COVID19, we find our score useful, especially on the fact that LDH, blood counts and X ray are easily available, even in low income emergency rooms, and poses a low risk to healthcare infection spread. We acknowledge that future evaluation of our proposed scores are needed.
ConclusionWe propose that clinicians use diagnostic tools to anticipate RT-PCR testing. This instrument encompassing WBC count, LDH, and image findings offers adequate sensitivity and specificity for this task.
ContributorsStudy design was idealized by TV, CF, BS and RS.
Data were collected by M Berger, CF, TV and PS and were reviewed by LA, PC, M Butzke and RZ.
Statistical analyses and predictive models were performed by TV. All investigators contributed equally in writing.
We would like to thank Jeffrey Hau for providing key insights on statistical analysis.
Conflicts of interestThe authors declare no conflicts of interest.