Paracoccidioidomycosis (PCM) is a fungal disease, chronic and endemic, especially in Latin America.1 Aiming to acquire more information about the effectiveness of the treatment employed, this study aimed to describe the profiles of patients with paracoccidioidomycosis and compare treatments used in patients with PCM cared for at the Clinic of Tropical Medicine of the “Conjunto Hospitalar de Sorocaba” (CHS) regarding efficacy, safety and adherence to treatment.
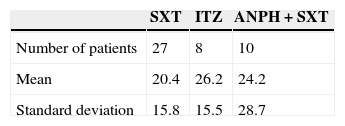
The present study evaluated treatment used and the profile of 45 patients with PCM diagnosis. We conducted a retrospective, epidemiological, quantitative, cross-sectional and observational study through analysis of data from medical records of 45 patients diagnosed with PCM. We found a patient profile very similar to that described in the literature, i.e., adult men (77.7%), aging 51.5 years.2 Regarding occupational activity, as in other studies, patients were mostly farmers (57.7%) or civil construction workers (11.1%).2 Three treatments evaluated were: trimoxazole {1600+800mg/day} (SXT), amphotericin B {1mg/kg/day} followed by trimoxazole for maintenance {1600+800mg/day} (SXT+ANPH), and itraconazole {200mg/day} (ITZ). To compare the treatments efficacy, each drug regimen was associated with clinical improvement (in days) defined as the absence of signs and symptoms and overall treatment time (in months) defined as a negative serology. The mean time to clinical improvement showed no difference between the three tested treatments (p=0.714). Table 1 shows the results.
Regarding the overall treatment time, the group treated with SXT finished treatment with medical discharge in 15.2 months. For the ANPH+SXT group, mean time was 13.3 months and for the ITZ, was 18.4 months. The differences were not statistically significant (p=0.592).
The mean time until seeking medical assistance was 2.13 months. For patients related to rural activities, time was 2.3 months, while for all others, it was 1.8 months (p=0.65). This finding is supported by other authors who reported that residents of rural areas have greater difficulty in accessing public health services, which contributes to late diagnosis of disease.3
Despite the consensus of the Brazilian Society of Tropical Medicine and other studies suggesting a greater efficacy of itraconazole, the present study, under the conditions it was held, did not confirm this result.1,4 No difference was found between the SXT combination, amphotericin B followed by SXT and ITZ with respect to signs of clinical improvement, symptoms, and time to negative serology. Compliance rate treatment in non-alcoholics was 79.3%, falling to 43.7% for frequent users of alcohol (p=0.02). Alcohol use was negatively related with treatment compliance, as was also found in other studies.5 The data show that treatment options outlined in the Brazilian Consensus for treatment of PCM, correctly used, showed similar results regarding clinical improvement and overall treatment time.
Conflicts of interestThe authors declare no conflicts of interest.