According to World Health Organization (WHO) estimates, about one million children annually develop tuberculosis (TB) worldwide, accounting for about 11% of all TB cases. Pediatric pulmonary TB (PTB) is often not considered a priority by national TB control programs, because limited resources restrict the focus of national TB control programs to only the most infectious sputum smear-positive cases who contribute to TB transmission. Considering the fact that children contribute a significant proportion to the global TB disease burden and suffer severe TB-related morbidity and mortality is growing, accurate and timely diagnosis is very necessary and crucial to control and treat pediatric PTB.1
The real-time PCR (RT-PCR) for TB is a rapid and reliable method for the diagnosis of PTB. Although TB RT-PCR provides high specificity (up to 100%), the sensitivity has a wide range (42.8–96.7%), depending on the results of smears, cultures and other clinical specimens.2,3 Young children are unable to expectorate sputum samples, so other clinical specimens may be the first choice for TB RT-PCR, including bronchoalveolar lavage fluid (BALF). To date, there has been no study investigating the diagnostic value of TB RT-PCR on BALF in the diagnosis of pediatric PTB. The aim of the present study was to evaluate the efficacy of the TB RT-PCR assay on BALF for diagnosing PTB in children and distinguishing it from other pulmonary diseases.
Between June 2008 and November 2012, patients aged <15 years were enrolled in this study. PTB patients were diagnosed based on history of TB contact, clinical symptoms, chest X-ray examination, tuberculin skin test, acid-fast bacilli (AFB) detection (Auramine O stain) and mycobacterial culture. BALF was performed according to the European Respiratory Society recommendations. BALF samples were then processed for AFB detection, TB RT-PCR analysis (TB RT-PCR kit, DAAN, China; LightCycler®480, Roche, Germany) and mycobacterial culture (Bact/Alert 3D, Biomerieux Durham, USA). The sensitivity and specificity for BALF TB RT-PCR assay were calculated and compared with other assays in diagnosing pediatric PTB. Differences in sensitivity and specificity between two tests were estimated by the McNemar's test, with p<0.05 considered significant. All calculations were estimated at a 95% confidence interval (95% CI). The protocol was approved by the Ethical Committee of the institute. Written informed consent was not required because of the retrospective nature of the investigation.
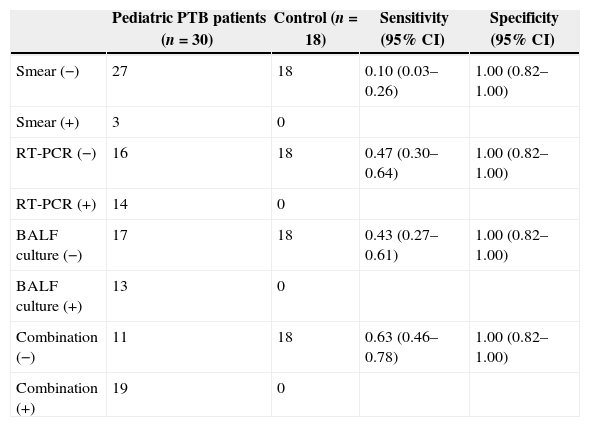
The mean age of all patients was 4.05 years (SD 4.34, range 0.25–14), and 64.6% were male. Among 30 pediatric PTB patients (mean age 2.74±3.45 years, range 0.33–14), 13 cases were mycobacterial culture positive, three cases were BALF smear positive TB cases. The mean age of the 18 non-PTB patients was 6.24 years (SD 4.86, range 0.25–13), and 77.8% of these patients were male. Table 1 shows that sensitivities of BALF AFB detection, RT-PCR, culture and combination of RT-PCR and culture were 0.10 (95% CI: 0.03–0.26), 0.47 (95% CI: 0.30–0.64), 0.43 (95% CI: 0.27–0.61), 0.63 (95% CI: 0.46–0.78), respectively. Specificities of the four methods were 1.00 (95% CI: 0.82–1.00). Compared with BALF AFB detection, BALF TB culture or BALF TB RT-PCR had significantly higher sensitivities (all p<0.05). Sensitivities of BALF TB culture and BALF TB RT-PCR were not significantly different (p>0.05). Combination of BALF TB culture and BALF TB RT-PCR was significantly better than each method individually (p<0.05).
Sensitivity and specificity of the four diagnostic methods for pediatric PTB.
| Pediatric PTB patients (n=30) | Control (n=18) | Sensitivity (95% CI) | Specificity (95% CI) | |
|---|---|---|---|---|
| Smear (−) | 27 | 18 | 0.10 (0.03–0.26) | 1.00 (0.82–1.00) |
| Smear (+) | 3 | 0 | ||
| RT-PCR (−) | 16 | 18 | 0.47 (0.30–0.64) | 1.00 (0.82–1.00) |
| RT-PCR (+) | 14 | 0 | ||
| BALF culture (−) | 17 | 18 | 0.43 (0.27–0.61) | 1.00 (0.82–1.00) |
| BALF culture (+) | 13 | 0 | ||
| Combination (−) | 11 | 18 | 0.63 (0.46–0.78) | 1.00 (0.82–1.00) |
| Combination (+) | 19 | 0 |
To the best of our knowledge, only two reports investigated the diagnostic value of PCR in BALF. One report from Switzerland showed a 78% positivity of TB PCR (AMPLICOR kits, Roche Diagnostics, Germany) in BALF in patients with culture-positive PTB.4 Only patients with smear- or culture-positive PTB were enrolled in the previous analysis. Thus, it is not surprising to obtain a high positive rate for a PCR that detects an M. tuberculosis DNA segment in patients who have high organism loads. Meanwhile, the usually paucibacillary nature of children TB could possibly justify the low rate of TB diagnosis using RT-PCR in BALF. Another report showed a 28.1% sensitivity of TB PCR (Multiplex TBC PCR-module, BIORON Diagnostics GmbH, Germany) in BALF and 99% specificity.5 The sensitivity was similar to that found in our study, suggesting that BALF RT-PCR is a moderate diagnostic test for pediatric PTB.
In summary, the sensitivity and specificity of BALF RT-PCR assay for diagnosing TB in children is a moderate diagnostic test and is more sensitive than conventional acid-fast staining techniques, and has equal sensitivity to mycobacterial culture. In addition, combination of BALF culture and TB RT-PCR had marked additive diagnostic value.
Conflicts of interestThe authors declare no conflicts of interest.
This work was supported by a grant from the Health Department of Shandong Province (no. 2011HZ085).