Life expectancy of people living with human immunodeficiency (PLHIV) has increased mainly due to the accessibility and effectiveness of antiretroviral therapy (ART). However, adverse effects from long-term use of antiretrovirals, and the physiological changes associated with aging, may compromise the quality of life of PLHIV, in addition to causing new demands on the healthcare system.
ObjectivesEstimate the frequency of osteoporosis and osteopenia in patients on prolonged ART and to verify their associated factors.
MethodsA cross-sectional study was conducted in Belo Horizonte, Minas Gerais, Brazil, from August 2017 to June 2018, in a sample of PLHIV (age ≥ 18 years) who started ART between 2001 and 2005. Data were collected through face-to-face interviews, physical evaluation, laboratory tests, and Dual-Energy X-Ray Absorptiometry Screening (DEXA). The outcome of interest was presence of bone alteration, defined as presence of osteopenia or osteoporosis in DEXA. The association between the explanatory variables and the event was assessed through odds ratio (OR) estimate, with 95% confidence interval (CI). Multiple logistic regression was performed to evaluate factors independently associated with bone alteration.
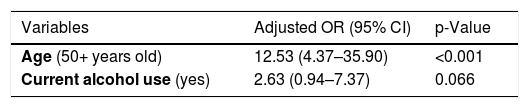
ResultsAmong 92 participants, 47.8% presented bone alteration (19.6% osteoporosis and 28.2% osteopenia). The variables that remained in the final logistic regression model were age ≥ 50 years (OR: 12.53; 95% CI: 4.37–35.90) and current alcohol use (OR: 2.63; 95% CI: 0.94–7.37).
ConclusionsThis study showed a high frequency of bone changes, especially in PLHIV older than 50 years. This information is useful to stimulate the screening and timely intervention of this comorbidity of PLHIV on prolonged use of ART in order to prevent or minimize complications and new demands on the healthcare system.
Antiretroviral therapy (ART) was introduced in the 1990s to control human immunodeficiency (HIV) replication in people living with HIV (PLHIV). As a result, an increase in expectancy and quality of life of PLHIV, and a significant decrease of HIV transmission in the general population were noticed. In addition, early initiation of ART contributes to immune recovery and prevents the occurrence of acquired immunodeficiency syndrome (AIDS) cases.1 However, to achieve this therapeutic success, the individual must regularly take ART which is based on at least three distinct antiretroviral drugs. According to the Brazilian HIV guidelines, the first choice for treatment should include a combination of two nucleoside analog reverse transcriptase inhibitors (lamivudine and tenofovir) associated with a non-nucleoside reverse transcriptase inhibitor (efavirenz) or an integrase inhibitor (dolutegravir; incorporated from 2017, becoming preferred over efavirenz).2
Between 2005 and 2015, there was a 45% reduction in HIV/AIDS mortality worldwide, which reflected an increase in the number of PLHIV (from 28 million to 38.8 million). In Brazil, the number of PLHIV increased from 490,000 in 2005 to 860,000 in 2017. This drop in mortality rates was mainly due to a significant increase in the proportion of PLHIV on ART worldwide, from 4.8% in 2005 to 40.5% in 2015.3,4 However, prolonged use of ART combined with the natural aging process may contribute to the occurrence of chronic diseases. Among the main comorbidities, metabolic alterations should be highlighted such as diabetes mellitus, dyslipidemia, liver disease, nephropathy, and bone changes including osteonecrosis of the femoral head, osteopenia, and osteoporosis.5,6
Osteopenia and osteoporosis are metabolic changes that lead to reduction of bone microarchitecture, bone fragility, and increased fracture risk.7,8 PLHIV present higher fracture risk than the general population. In addition, PLHIV may present other risk factors associated with development of fractures in early ages, such as presence of multiple comorbidities, multiple drug use, peripheral neuropathy, and frailty.9–11
Despite the evidence that prolonged ART use leads to bone changes, this subject is poorly investigated in the Brazilian context. In an open cohort study with 108 PLHIV in Brazil, mean age 43 years and mean time on ART 5.2 years, the prevalence of low bone mineral density (BMD) was 23.2%.12 When stratified by age, 54% of patients over 50 years old showed decreased BMD compared to only 15% in younger patients. In a cross-sectional study in 300 patients with similar mean age and time on ART, the general prevalence of decreased BMD was 54.7%, with higher prevalence among people aged 50 or over (73.7%).13 Other studies in the US found high proportions of bone alteration in PLHIV, with a 2–6% decline in BMD during the first years of ART use, regardless of the antiretroviral regimen.14–17 This significant bone loss resembles the population with chronic glucocorticoid use or the first year after menopause.18,19
Use of the antiretroviral tenofovir (TDF) has been more associated with increased bone loss when compared to other antiretroviral drugs.20 Prolonged TDF exposure contributes to increased parathyroid hormone (PTH) activity, which decreases calcium concentration in bone tissue while increasing plasma concentration.21 Actually, there is evidence that bone loss occurs with all ART regimens, possibly due to increased bone catabolism after viral suppression.22–26
Despite the knowledge about the risk of bone alteration, further contextualized studies are needed in the Brazilian HIV population, in order to investigate the impact on both quality of life and the healthcare system. In addition, guidelines are needed to address screening and preventive actions. The current knowledge is not satisfactorily applied in clinical practice, possibly due to lack of awareness and financial support in this issue. In this perspective, this study aimed to estimate the frequency of osteopenia and osteoporosis and their associated factors in a sample of PLHIV on prolonged ART use.
MethodsStudy design and populationThis was a cross-sectional study conducted in the city of Belo Horizonte, Minas Gerais state, Brazil, from August 2017 to June 2018. This study included patients from a historical cohort that aimed to evaluate adverse effects on prolonged ART use in PLHIV (≥ 18 years) who started ART between 2001 and 2005 and continued to receive regular care in a public referral center until 2017/2018.27
Outcome of interest and explanatory variablesThe outcome of interest was presence of bone alteration (osteopenia or osteoporosis) diagnosed by the Dual-Energy X-Ray Absorptiometry (DEXA) method and classified according to BMD level. BMD classification was based on the World Health Organization (WHO) international standards. For individuals aged 50 years and over, the T score was: a) DEXA T score > −1 = absence of bone alteration; b) DEXA T score between −2.5 and −1.0 = osteopenia; and c) DEXA T score <−2.5 = osteoporosis. On the other hand, for individuals under 50 years of age, DEXA Z score < of −2.0 indicates bone change (without sub-classifications).7,8,28
Potentially explanatory variables were sociodemographic, clinical, lifestyle and dietary data, current use (yes or no) of alcohol, tobacco and illicit drugs, medication use, and healthcare system use. ART use was dichotomized by the median time of use. The level of physical activity was assessed by Baecke's questionnaire and categorized as insufficient (score < 8.0) or sufficient (score ≥ 8.0) levels.29 This instrument evaluates habitual physical activity (at work, at leisure, or in locomotion activities) over the previous 12 months and had been validated in Brazil to the general and HIV population.30 The reference values for biochemical exams were: a) calcium: 8.5–9.5 mg/dL; b) PTH: 15–68.3 pg/mL; c) vitamin D: > 30 ng/mL; d) phosphorus: 2.5–4.5 mg/dL; and e) creatinine: 0.7–1.0 mg/dL. HIV viral load (VL) was classified as detectable or undetectable (< 40 copies/mL), and TCD4+ lymphocyte count was categorized using 500 cells/mm³ as the cut-off value. Body mass index (BMI) was calculated by anthropometric data registered during the interviews and classified according to WHO recommendations as normal (18.5–24.9 kg/m²), underweight (< 18.4 kg/m²) or overweight (≥ 25.0 kg/m²).31
Recruitment and data collectionThe recruitment of eligible patients was performed in-person at the public referral center, during medical appointments or ART dispensation. Patients were invited to participate in the study and, in case of agreement, to sign the Informed Consent Form. Face-to-face interviews were conducted using a semi-structured questionnaire. After the interview, weight and height were measured using a properly calibrated anthropometric scale. Biochemical examinations were performed in the clinical pathology laboratory of the Federal University of Minas Gerais (UFMG) and bone densitometry (DEXA) was assessed in a private laboratory (GE HealthCare - Lunar Prodigy Advance - PA + 130,267). The test results were attached to the participants' medical records and a copy delivered to them at the time of ART dispensation. The data were collected through Questionnaire Development System software, version 2.6.1.1.
Statistical analysisDescriptive analyses were performed to characterize the study population. The difference between the proportions of the explanatory variables and outcome was assessed by Pearson's chi-square test in which variables presenting p-value <0.25 were included in the initial multivariate model. Variables presenting p-value <0.05 and those with epidemiological relevance were considered for the final logistic regression model. The magnitude of associations was estimated using odds ratio (OR), with 95% confidence interval (CI). Statistical analysis was performed using EpiInfo® version 7.2.2.6 and SAS® software version 9.2.
Ethical considerationsThis study was approved by the Research Ethics Committee of the Federal University of Minas Gerais (CAAE Number: 62710316.8.0000.5149) and by the Municipal Health Secretariat of the city of Belo Horizonte. All participants signed the Informed Consent Form prior to beginning data collection.
ResultsOut of the 204 individuals included in the cohort study,27 45 (22.0%) did not participate in the current study due to death, treatment abandonment, or transfer to other healthcare services, and 48 (23.5%) could not be located. Among the 111 invited patients, 17 (15.3%) declined the invitation and 2 (1.8%) have turned in the DEXA exam. Thus, 92 participants were left for this analysis.
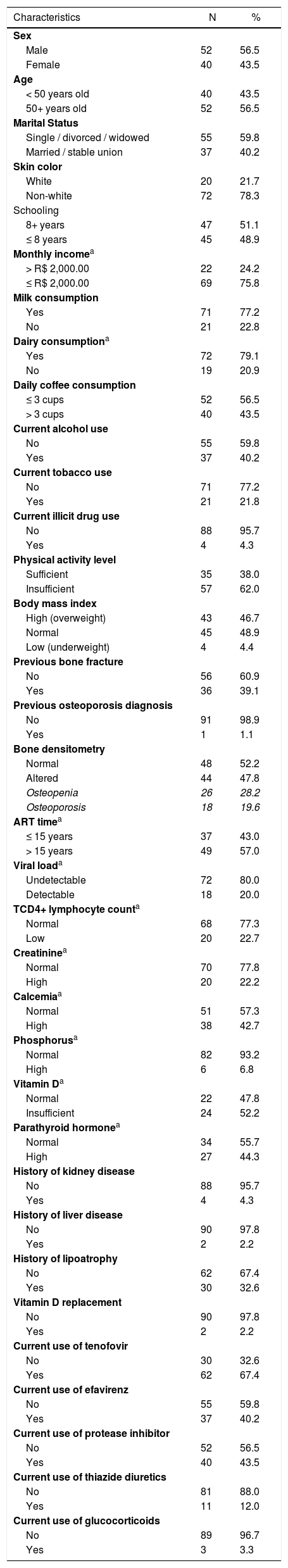
Mean age was 52 years (56.5% > 50 years old), and most participants were male (56.5%), single, divorced or widowed (59.8%), non-white (78.3%), had more than eight years of education (51.1%) and had an income less than or equal to R$ 2,000.00 (two thousand reais) (75.8%). The majority reported consuming milk (77.2%) or dairy products (79.1%) and three or more cups of coffee daily (43.5%). Regarding drug use, 40.2% consumed alcohol, 22.8% tobacco and 4.4% illicit drugs (Table 1).
Sociodemographic, clinical and laboratory profile of treatment-experienced PLHIV (N = 92).
| Characteristics | N | % |
|---|---|---|
| Sex | ||
| Male | 52 | 56.5 |
| Female | 40 | 43.5 |
| Age | ||
| < 50 years old | 40 | 43.5 |
| 50+ years old | 52 | 56.5 |
| Marital Status | ||
| Single / divorced / widowed | 55 | 59.8 |
| Married / stable union | 37 | 40.2 |
| Skin color | ||
| White | 20 | 21.7 |
| Non-white | 72 | 78.3 |
| Schooling | ||
| 8+ years | 47 | 51.1 |
| ≤ 8 years | 45 | 48.9 |
| Monthly incomea | ||
| > R$ 2,000.00 | 22 | 24.2 |
| ≤ R$ 2,000.00 | 69 | 75.8 |
| Milk consumption | ||
| Yes | 71 | 77.2 |
| No | 21 | 22.8 |
| Dairy consumptiona | ||
| Yes | 72 | 79.1 |
| No | 19 | 20.9 |
| Daily coffee consumption | ||
| ≤ 3 cups | 52 | 56.5 |
| > 3 cups | 40 | 43.5 |
| Current alcohol use | ||
| No | 55 | 59.8 |
| Yes | 37 | 40.2 |
| Current tobacco use | ||
| No | 71 | 77.2 |
| Yes | 21 | 21.8 |
| Current illicit drug use | ||
| No | 88 | 95.7 |
| Yes | 4 | 4.3 |
| Physical activity level | ||
| Sufficient | 35 | 38.0 |
| Insufficient | 57 | 62.0 |
| Body mass index | ||
| High (overweight) | 43 | 46.7 |
| Normal | 45 | 48.9 |
| Low (underweight) | 4 | 4.4 |
| Previous bone fracture | ||
| No | 56 | 60.9 |
| Yes | 36 | 39.1 |
| Previous osteoporosis diagnosis | ||
| No | 91 | 98.9 |
| Yes | 1 | 1.1 |
| Bone densitometry | ||
| Normal | 48 | 52.2 |
| Altered | 44 | 47.8 |
| Osteopenia | 26 | 28.2 |
| Osteoporosis | 18 | 19.6 |
| ART timea | ||
| ≤ 15 years | 37 | 43.0 |
| > 15 years | 49 | 57.0 |
| Viral loada | ||
| Undetectable | 72 | 80.0 |
| Detectable | 18 | 20.0 |
| TCD4+ lymphocyte counta | ||
| Normal | 68 | 77.3 |
| Low | 20 | 22.7 |
| Creatininea | ||
| Normal | 70 | 77.8 |
| High | 20 | 22.2 |
| Calcemiaa | ||
| Normal | 51 | 57.3 |
| High | 38 | 42.7 |
| Phosphorusa | ||
| Normal | 82 | 93.2 |
| High | 6 | 6.8 |
| Vitamin Da | ||
| Normal | 22 | 47.8 |
| Insufficient | 24 | 52.2 |
| Parathyroid hormonea | ||
| Normal | 34 | 55.7 |
| High | 27 | 44.3 |
| History of kidney disease | ||
| No | 88 | 95.7 |
| Yes | 4 | 4.3 |
| History of liver disease | ||
| No | 90 | 97.8 |
| Yes | 2 | 2.2 |
| History of lipoatrophy | ||
| No | 62 | 67.4 |
| Yes | 30 | 32.6 |
| Vitamin D replacement | ||
| No | 90 | 97.8 |
| Yes | 2 | 2.2 |
| Current use of tenofovir | ||
| No | 30 | 32.6 |
| Yes | 62 | 67.4 |
| Current use of efavirenz | ||
| No | 55 | 59.8 |
| Yes | 37 | 40.2 |
| Current use of protease inhibitor | ||
| No | 52 | 56.5 |
| Yes | 40 | 43.5 |
| Current use of thiazide diuretics | ||
| No | 81 | 88.0 |
| Yes | 11 | 12.0 |
| Current use of glucocorticoids | ||
| No | 89 | 96.7 |
| Yes | 3 | 3.3 |
Almost half of the participants were overweight, 4.4% underweight, and 62.0% had insufficient physical activity. The minimum time of ART use was 12 years, the maximum 17 years and the average time was 15 years (57.0% of participants had been on ART > 15 years). At the time of the interview, 67.4% had been taking TDF, 40.2% efavirenz and 43.5% protease inhibitor drugs. In addition, 12% had been taking thiazide diuretics, 3.3% glucocorticoids, and 2.2% vitamin D drug replacement (Table 1).
Out of the participants with available tests, 44.3% presented high PTH plasma levels, 52.2% insufficient vitamin D, 42.7% high calcium level, 22.2% high creatinine, and 6.8% high phosphorus levels. Most participants had undetectable VL (80.0%) and a TCD4+ lymphocyte count greater than 500 cells/mm3 (77.3%). Concerning clinical diagnoses, 4.3% reported kidney and 2.1% liver diseases; and in 32.6% of the patients, lipoatrophy was diagnosed or noticed by the patient in some part of the body (Table 1).
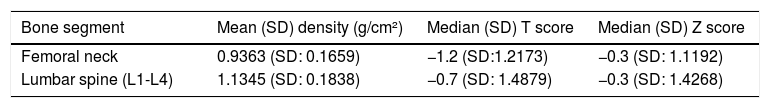
Forty-four (47.8%) participants presented the outcome (28.2% osteopenia and 19.6% osteoporosis in DEXA), 36 (39.1%) reported a previous history of fracture. Only one participant had been diagnosed with osteoporosis (Table 1). Mean BMD of the femoral neck was 0.9363 g/cm² (standard deviation [SD]: 0.1659), and 1.1345 g/cm² (SD: 0.1838) in the lumbar spine (L1-L4). The median T score and Z scores were -1.2 (SD: 1.22) and -0.3 (SD: 1.12) for the femur, and -0.7 (SD: 1, 49) and -0.3 (SD: 1.43) for the lumbar spine, respectively (Table 2).
Evaluation of DEXA quantitative variables in treatment-experienced PLHIV (N = 92).
| Bone segment | Mean (SD) density (g/cm²) | Median (SD) T score | Median (SD) Z score |
|---|---|---|---|
| Femoral neck | 0.9363 (SD: 0.1659) | −1.2 (SD:1.2173) | −0.3 (SD: 1.1192) |
| Lumbar spine (L1-L4) | 1.1345 (SD: 0.1838) | −0.7 (SD: 1.4879) | −0.3 (SD: 1.4268) |
SD: Standard Deviation; g: grams; cm: centimeters.
Interpretation.
50+ years old.
T score ≥ -1.0: no significant bone alteration.
T score between −1.0 and −2.5: osteopenia.
T score ≤ −2.5: osteoporosis.
< 50 years old.
Z score ≤ −2.0: altered (without sub classifications).
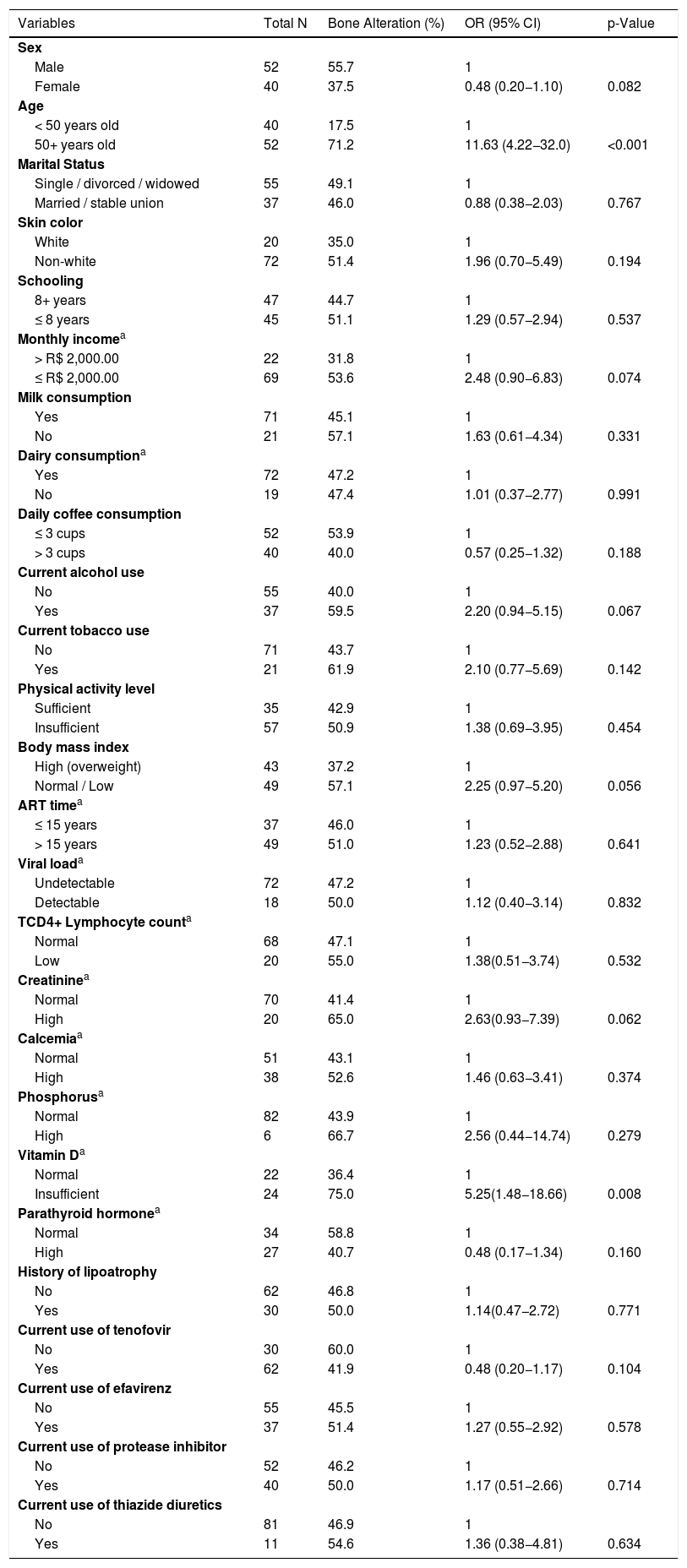
Univariate analysis showed that 71.2% of those older than 50 years presented bone alteration, compared to 17.5% of younger individuals (OR: 11.63; 95% CI: 4.22–32.0; p-value <0.001). A total of 57.1% of participants with normal or underweight BMI (OR: 2.25; 95% CI: 0.97−5.20; p = 0.056) and 75.0% of those with altered vitamin D levels (OR: 5.25; 95% CI: 1.48−18.66; p = 0.008) had bone alteration (Table 3). The variables age ≥ 50 years (OR: 12.53; 95% CI: 4.37–35.90) and current alcohol use (OR: 2.63; 95% CI: 0.94–7.37) remained in the final multivariate model (Table 4).
Factors associated with bone alteration in treatment-experienced PLHIV, Univariate Analysis (N = 92).
| Variables | Total N | Bone Alteration (%) | OR (95% CI) | p-Value |
|---|---|---|---|---|
| Sex | ||||
| Male | 52 | 55.7 | 1 | |
| Female | 40 | 37.5 | 0.48 (0.20−1.10) | 0.082 |
| Age | ||||
| < 50 years old | 40 | 17.5 | 1 | |
| 50+ years old | 52 | 71.2 | 11.63 (4.22−32.0) | <0.001 |
| Marital Status | ||||
| Single / divorced / widowed | 55 | 49.1 | 1 | |
| Married / stable union | 37 | 46.0 | 0.88 (0.38−2.03) | 0.767 |
| Skin color | ||||
| White | 20 | 35.0 | 1 | |
| Non-white | 72 | 51.4 | 1.96 (0.70−5.49) | 0.194 |
| Schooling | ||||
| 8+ years | 47 | 44.7 | 1 | |
| ≤ 8 years | 45 | 51.1 | 1.29 (0.57−2.94) | 0.537 |
| Monthly incomea | ||||
| > R$ 2,000.00 | 22 | 31.8 | 1 | |
| ≤ R$ 2,000.00 | 69 | 53.6 | 2.48 (0.90−6.83) | 0.074 |
| Milk consumption | ||||
| Yes | 71 | 45.1 | 1 | |
| No | 21 | 57.1 | 1.63 (0.61−4.34) | 0.331 |
| Dairy consumptiona | ||||
| Yes | 72 | 47.2 | 1 | |
| No | 19 | 47.4 | 1.01 (0.37−2.77) | 0.991 |
| Daily coffee consumption | ||||
| ≤ 3 cups | 52 | 53.9 | 1 | |
| > 3 cups | 40 | 40.0 | 0.57 (0.25−1.32) | 0.188 |
| Current alcohol use | ||||
| No | 55 | 40.0 | 1 | |
| Yes | 37 | 59.5 | 2.20 (0.94−5.15) | 0.067 |
| Current tobacco use | ||||
| No | 71 | 43.7 | 1 | |
| Yes | 21 | 61.9 | 2.10 (0.77−5.69) | 0.142 |
| Physical activity level | ||||
| Sufficient | 35 | 42.9 | 1 | |
| Insufficient | 57 | 50.9 | 1.38 (0.69−3.95) | 0.454 |
| Body mass index | ||||
| High (overweight) | 43 | 37.2 | 1 | |
| Normal / Low | 49 | 57.1 | 2.25 (0.97−5.20) | 0.056 |
| ART timea | ||||
| ≤ 15 years | 37 | 46.0 | 1 | |
| > 15 years | 49 | 51.0 | 1.23 (0.52−2.88) | 0.641 |
| Viral loada | ||||
| Undetectable | 72 | 47.2 | 1 | |
| Detectable | 18 | 50.0 | 1.12 (0.40−3.14) | 0.832 |
| TCD4+ Lymphocyte counta | ||||
| Normal | 68 | 47.1 | 1 | |
| Low | 20 | 55.0 | 1.38(0.51−3.74) | 0.532 |
| Creatininea | ||||
| Normal | 70 | 41.4 | 1 | |
| High | 20 | 65.0 | 2.63(0.93−7.39) | 0.062 |
| Calcemiaa | ||||
| Normal | 51 | 43.1 | 1 | |
| High | 38 | 52.6 | 1.46 (0.63−3.41) | 0.374 |
| Phosphorusa | ||||
| Normal | 82 | 43.9 | 1 | |
| High | 6 | 66.7 | 2.56 (0.44−14.74) | 0.279 |
| Vitamin Da | ||||
| Normal | 22 | 36.4 | 1 | |
| Insufficient | 24 | 75.0 | 5.25(1.48−18.66) | 0.008 |
| Parathyroid hormonea | ||||
| Normal | 34 | 58.8 | 1 | |
| High | 27 | 40.7 | 0.48 (0.17−1.34) | 0.160 |
| History of lipoatrophy | ||||
| No | 62 | 46.8 | 1 | |
| Yes | 30 | 50.0 | 1.14(0.47−2.72) | 0.771 |
| Current use of tenofovir | ||||
| No | 30 | 60.0 | 1 | |
| Yes | 62 | 41.9 | 0.48 (0.20−1.17) | 0.104 |
| Current use of efavirenz | ||||
| No | 55 | 45.5 | 1 | |
| Yes | 37 | 51.4 | 1.27 (0.55−2.92) | 0.578 |
| Current use of protease inhibitor | ||||
| No | 52 | 46.2 | 1 | |
| Yes | 40 | 50.0 | 1.17 (0.51−2.66) | 0.714 |
| Current use of thiazide diuretics | ||||
| No | 81 | 46.9 | 1 | |
| Yes | 11 | 54.6 | 1.36 (0.38−4.81) | 0.634 |
In this study, the frequency of osteopenia and osteoporosis was considerably higher than in the general Brazilian population (ranging from 4.4%–27.4%).32,33 However, these rates may be comparable to those of the elderly population (over 65 years old) in Brazil, which ranges from 33.3–57.4%.34 Considering other studies in the HIV population, these results are similar to that found by Pinto Neto et al., in which 54.8% had bone alteration, and by Escota et al., who reported 61% alteration in a population on ART use for at least four years.13,14 Although for some authors the prevalence and factors associated with osteoporosis in the Brazilian population are unclear, results of this study show that bone abnormalities are manifested earlier and more frequently in PLHIV on ART use compared to the general population. These findings underscore the importance of a specific approach to these changes aiming at reducing fragility fractures, complications, and generating new demands for the healthcare system.35
The frequency of osteoporosis found in the present study (19.6%), with a mean age of 52 years, was higher than the frequency found by Mary-Krause et al. (14.6%), with a mean age of 46 years.20 In addition, the mean lumbar spine and femoral neck BMD found in the present study are close to the results found in a Brazilian study with a sample of older people.36 This evidence corroborates the hypothesis that there is an earlier incidence and higher prevalence of bone disease among PLHIV.
As shown, a positive and significant association was observed between older age and bone alteration. Age is a major risk factor for declining BMD in both PLHIV and the general population. However, it is observed that this decline affects PLHIV on ART earlier than the general population. Kanis et al. reported that the prevalence of osteoporosis in PLHIV under 50 years old corresponds to the prevalence in European men over 75 years.37 The European Clinical AIDS Society recommends screening for bone alteration by DEXA in PLHIV over 50 years in men, postmenopausal women, and adults with risk factors such as history of fragility fracture, glucocorticoid treatment for more than three months, or high risk of falls.38,39 Given these results, tracking bone alteration following these recommendations seems to be the best way to avoid fractures and other complications.
In univariate analysis, low vitamin D levels were associated with bone alteration, despite the large number of individuals with missing vitamin D results. Adequate absorption of calcium, an important element in bone remineralization, requires enough levels of vitamin D to stimulate its absorption from the intestine. Other effects of vitamin D action include regulation of bone reabsorption, enhancing calcium reabsorption in the distal renal tubules, and repression of PTH transcription and secretion.40,41 As a result, the bone health guidelines for PLHIV advise considering intervention with bisphosphonate drugs, in addition to calcium and vitamin D replacement in cases of prior fracture or DEXA showing T score below −2.5 in any segment.39 In a 48-week randomized clinical trial with 165 adults on ART, it was observed that calcium and vitamin D supplementation could attenuate bone decline.42 However, further studies are needed to evaluate the preventive effect of calcium and vitamin D supplementation among PLHIV on ART in Brazil. Thus, according to the DEXA results shown in this study, 22 (25%) participants would have an effective indication for treatment.
The association between bone alteration and normal or low BMI found in this study seems to corroborate the results of other studies. Pinto Neto et al. found a similar association, although with greater magnitude (OR: 5.7 and 12.0, for normal and low BMI, respectively).13 Mary-Krause et al. complement this association by recommending screening for men under 60 years of age and BMI < 20 kg/m².20 A meta-analysis with 10 studies totaling 1371 HIV positive and 1644 negative adults (age and sex adjusted) observed that PLHIV presented a mean weight of 5.1 kg lower than HIV negative controls (p < 0.001).43 Unadjusted analyses found significant lower BMD in all bone segments in PLHIV (p < 0.01). After adjustment for body weight, the association remained significant for total hip and femoral neck. Multifactorial determinants may cause bone decline in PLHIV, some of which are reversible. However, aging affects directly the bone changes and also muscle tissue, causing sarcopenia, which will directly influence bone tissue maintenance. Recommendations for lifestyle changes such as weight-bearing muscle strengthening and nutritional monitoring should be accessible to this population.
The multivariate analysis identified a positive independent association between current alcohol consumption and bone alterations. These results were corroborated by other studies. Tian et al. reported almost two-fold greater chance of bone alteration in postmenopausal women and elderly men with alcohol habits.44 In addition, results from a meta-analysis also identified alcohol consumption as risk factor for bone mineral decline.45 A population-based study conducted in Norway found higher risk of hip fracture in individuals with insufficient physical activity, history of smoking, and excessive alcohol consumption.46 Thus, greater attention, guidance and screening should be offered especially to PLHIV on ART presenting other risk factors such as alcohol use.
PLHIV mortality rates decreased substantially after the introduction of highly effective combined ART, resulting in aging of this population, which is increasingly affected by age-related non-communicable conditions.47 Several studies indicate a higher prevalence and earlier incidence of chronic diseases in PLHIV, compared to age-matched seronegative individuals, regardless of sex.48,49 In this study, as already mentioned, despite the high frequency of bone alteration, only one participant reported previous osteoporosis diagnosis. This result is worrisome, showing lack of tracking and awareness concerning the problem, which can be preventable through lifestyle changes.
In this study no association was found between use of TDF and efavirenz with the presence of bone changes, probably due to the sample size and design limitations, which have neither allowed the estimation of time on drug exposure nor to establish a logical temporal exposure. However, the literature shows a positive association. A published meta-analysis found that although the proportion of individuals treated with protease inhibitors or TDF with osteopenia or osteoporosis was higher when compared to their respective controls, although these results did not reach statistical significance.45 Thus, it is still unclear how and which drugs are truly associated with declining BMD, requiring more specific, longer, and more robust additional studies to conclude and refine future screening and management approaches and recommendations.
This study had some limitations, especially related to the sample size and the lack of complete information regarding laboratory results, reducing the study power. For this reason, some variables were analyzed in a dichotomous way and it was not possible to make stratified analysis by sex or age. The information was obtained from a sample of 92 patients followed-up in a single referral service, which might not represent the population of PLHIV on long-term ART use in the municipality. Thus, further studies with a larger sample and different conditions are needed to better evaluate the magnitude and factors associated with bone alteration in this population. However, despite these limitations, it was possible to notice a high frequency of bone alteration – osteoporosis and osteopenia – in this sample of PLHIV long time exposed to antiretroviral drugs, which reinforces the recommendation for routine bone alteration screening by densitometry in the SUS, as well as preventive measures, and when necessary, pharmacological treatment, in order to prevent or minimize complications and new demands for healthcare services.
Financing sourceThis work received financial support from the Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq (Process No. 474547-2013-2), from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES (postgraduate scholarship), and from the Fundação de Amparo à Pesquisa do Estado de Minas Gerais – FAPEMIG (postgraduate scholarship).
Declarations of interestThe authors declare no conflicts of interest.