Chronic granulomatous disease (CGD) is one of the most common genetic disorders of phagocytic functions, resulting from defective generation of the respiratory burst in human.1,2 Herein is described the case of a patient who was referred to this center with tuberculous meningitis (TBM), where upon further investigation during the course of disease confirmed the underlying diagnosis of CGD.
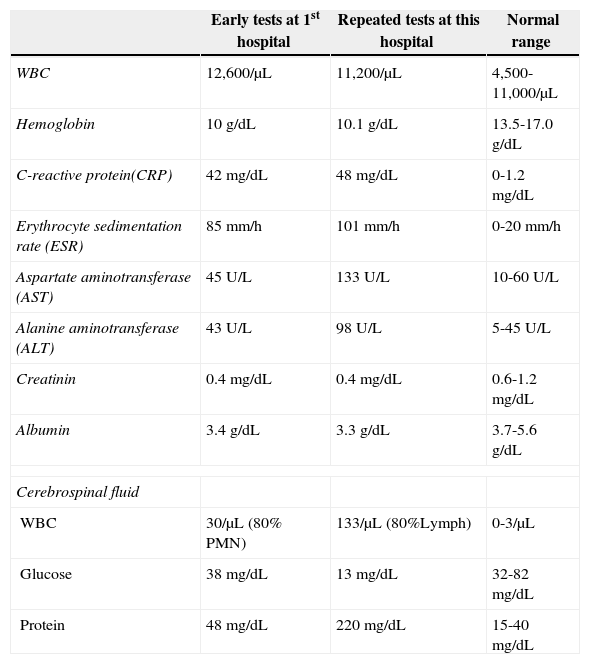
A 3 year-old girl was admitted to a general hospital in Western Iran due to persistent fever and chills that had lasted for three weeks. After an initial diagnosis of fever of unknown origin (FUO), laboratory testing was performed (Table 1). Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were both elevated. Hepatic enzymes were slightly increased. The patient had hypochromic microcytic anemia (Hb=10g/dL, MCV=69, MCH=22.9, reticulocyte=1.8%). Chest X-ray revealed two calcified lymph nodes in the right axillary region, ipsilateral to the site of the bacillus Calmette-Guérin (BCG) vaccination.
Laboratory findings of the chronic granulomatous disease patient with tuberculous meningitis.
| Early tests at 1st hospital | Repeated tests at this hospital | Normal range | |
|---|---|---|---|
| WBC | 12,600/μL | 11,200/μL | 4,500-11,000/μL |
| Hemoglobin | 10 g/dL | 10.1 g/dL | 13.5-17.0 g/dL |
| C-reactive protein(CRP) | 42 mg/dL | 48 mg/dL | 0-1.2 mg/dL |
| Erythrocyte sedimentation rate (ESR) | 85 mm/h | 101 mm/h | 0-20 mm/h |
| Aspartate aminotransferase (AST) | 45 U/L | 133 U/L | 10-60 U/L |
| Alanine aminotransferase (ALT) | 43 U/L | 98 U/L | 5-45 U/L |
| Creatinin | 0.4 mg/dL | 0.4 mg/dL | 0.6-1.2 mg/dL |
| Albumin | 3.4 g/dL | 3.3 g/dL | 3.7-5.6 g/dL |
| Cerebrospinal fluid | |||
| WBC | 30/μL (80% PMN) | 133/μL (80%Lymph) | 0-3/μL |
| Glucose | 38 mg/dL | 13 mg/dL | 32-82 mg/dL |
| Protein | 48 mg/dL | 220 mg/dL | 15-40 mg/dL |
Broad-spectrum antibiotics, such as vancomycin and ceftriaxone, were administered. However, the patient developed headache and vomiting. Following atonic seizures, she was moved to the intensive care unit (ICU). Phenobarbital and phenytoin were given to the patient. Lumbar puncture (LP) was performed due to suspicion of meningitis. Cerebrospinal fluid (CSF) examination revealed pleocytosis with predominance of polymorphonuclears (PMNs) (Table 1), while the presence of two acid-fast bacilli was reported in the CSF. Four drugs were added to the treatment protocol: isoniazid, rifampin, pyrazinamide, and ethambutol.
Due to persistent vomiting, fever, deterioration of the health status, and non-compliance of the patient with the treatment, the patient was referred to the Children's Medical Center, Pediatrics Center of Excellence, in Tehran, Iran. The laboratory tests were repeated, and a new LP was performed, which revealed lymphocytic pleocytosis with presence of three acid-fast bacilli (Table 1). The protein and glucose profiles of the CSF were consistent with TBM. Regarding the LP, anti–tuberculosis (anti-TB) agents were given to the patient with the addition of streptomycin instead of pyrazinamide, and vancomycin and ceftriaxone were discontinued. Computed tomography (CT) scan showed a mild ventriculomegaly; obstructive hydrocephaly due to the TBM was suggested based on these findings. Dexamethason and acetazolamide were given to reduce the meningeal inflammation.
Due to the suspicion of immunodeficiency, immunologic laboratory tests were performed. Immunoglubins and complement, anti-tetanus and anti-diphtheria seroprotection titers, and B- and T-cell enumeration were all in normal range. The phagocytic cell function was tested with a nitroblue-tetrazolium (NBT) slide test, which was defective. NBT was repeated and flow cytometric dihydrorhodamine 123 (DHR) assay was also performed, and the results were compatible with the diagnosis of CGD. Then, the patient was treated with interferon-γ as well as co-trimoxazole and itraconazole as prophylactic antibiotics and antifungals. After five days, symptoms including fever improved, except for headache and vomiting. Due to consistent headache, a ventriculoperitoneal shunting was performed, resulting in dramatically improvement of headache and vomiting. After three days, fever and all of the symptoms improved; the patient was discharged with good condition. Anti-TB drugs were continued for nine months, while the patient was prescribed INF-γ and prophylactic antifungals and antibiotics.
Patients with CGD are prone to life-threatening bacterial and fungal infections due to a defect in intracellular elimination of microorganisms. NADPH oxidase complex of phagocytic cells is a very important component in the defense against catalase positive bacteria such as Staphylococcus aureus, Serratia marcescens, and Aspergillus spp. However, the susceptibility to mycobacterial infections has always been questioned. Although experimental studies have demonstrated no difference between neutrophils of CGD patients and healthy subjects in destroying M. tuberculosis, recent studies have showed more evidence of the possible role of oxidative burst in host defense against mycobacterial infections.3
The diagnosis of TBM is challenging, especially in patients with underlying immunodeficiency. In the present case, the CGD diagnosis had an important role in successful treatment, although delayed. Unfortunately, physician unawareness is still an obstacle to timely diagnosis.4 There were some alerting clues for the suspicion of CGD, which should have been noted. History of unknown deaths in the family and consanguinity of parents should alert the physicians for possible primary immunodeficiency, as the results of misdiagnosis can be devastating.5 Prompt diagnosis of underlying immunodeficiency and appropriate treatments are the key elements that can save immunodeficient patients and avoid irreversible organ damage and death.
Conflict of interestAll authors declare to have no conflict of interest.