Despite the efforts made worldwide to reduce the number of cases of drug-susceptible tuberculosis, multidrug-resistant tuberculosis (MDR-TB) constitutes an important public health issue. Around 440,000 new cases of MDR-TB are estimated annually, although in 2008 only 7% of these (29,423 cases) were notified. The laboratory tests for diagnosing resistance may be phenotypic (based on culture growth in the presence of drugs) or genotypic (i.e. identification of the presence of mutations that confer resistance). The urgent need for a rapid means of detecting resistance to anti-TB drugs has resulted in the development of many genotypic methods over recent years. The treatment of MDR-TB is expensive, complex, prolonged (18–24 months) and associated with a higher incidence of adverse reactions. Some basic principles must be observed when prescribing an adequate treatment regimen for MDR-TB: (a) the association of at least four drugs (three of which should not have been used previously); (b) use of a fluoroquinolone; and (c) use of an injectable anti-TB drug. In Brazil, the therapeutic regimen for MDR-TB has been standardized and consists of five drugs: terizidone, levofloxacin, pyrazinamide, ethambutol and an aminoglycoside (streptomycin or amikacin). Pulmonary resection is an important tool in the coadjuvant treatment of MDR-TB. While a recent meta-analysis revealed an average cure rate of MDR-TB of 69%, clinical studies are currently being conducted with new drugs and with drugs already available on the market but with a new indication for TB, with encouraging results that will enable more effective treatment regimens to be planned in the future.
The emergence of drug-resistant tuberculosis (TB) is often attributed to a failure to implement adequate control programs for tuberculosis and to appropriately manage cases of the disease. Therefore, resistance to TB accurately reflects the poor quality of control programs and is a direct consequence of poor therapeutic practices with respect to the use of anti-TB drugs.1,2
Despite the efforts made worldwide to reduce the number of cases of drug-susceptible tuberculosis (TB),3 multidrug-resistant tuberculosis (MDR-TB) constitutes a public health problem and represents a severe obstacle to the control of TB, particularly in countries in which the prevalence of this disease remains high.4 Over the past two decades, various publications have registered the emergence of MDR-TB worldwide. MDR-TB is defined as cases of tuberculosis that are resistant to at least rifampicin and isoniazid.5–8
In 2008, 29,423 new cases of MDR-TB were notified worldwide in the 127 countries in which at least one case of this form of the disease was reported to the World Health Organization (WHO). These figures account for only 7% of the 440,000 new cases of MDR-TB estimated for that year and, of these, only one-fifth (1.2% of the total number) was treated in accordance with the WHO recommendations. Around 50% of the global burden of MDR-TB is concentrated in two countries, India and China, followed by Russia (9%). The WHO estimates that around 150,000 deaths occurred in patients with MDR-TB in 2008.9 The difference between the number of estimated cases and the number of treated cases should be interpreted as potential disseminating sources of multidrug-resistant strains and may be responsible for the increase in the number of cases.
More recently, cases have been described of extensively drug-resistant tuberculosis (XDR-TB) in which Mycobacterium tuberculosis is resistant to rifampicin, isoniazid, a fluoroquinolone and a second-line injectable drug (capreomycin, kanamycin or amikacin). An epidemic of XDR-TB was described for the first time in KwaZulu-Natal in South Africa in a population of HIV/AIDS patients, where the mean survival time between diagnosis by sputum smear microscopy and death was 16 days.10
According to a recent document issued by the WHO, 963 cases of XDR-TB were reported in 2008 worldwide, these data referring to all of the 41 countries in which at least one case was notified.9 Only 6 of these 41 countries (15%) reported more than 10 cases of XDR-TB. The proportion of cases of MDR-TB that are in fact XDR-TB exceeds 10% in the following countries: Estonia (19.7%), Latvia (15.1%), South Africa (10.5%) and Tajikistan (21%).11 Nevertheless, considering the difficulty in performing susceptibility tests for second-line drugs in routine services in the majority of countries, these data are in fact underestimated.
The worldwide trend of anti-TB drug resistance can be estimated for only 59 countries in which more than one investigation of resistance was performed between 1994 and 2009.9 Over the last decade in Brazil, an increase has occurred in primary isoniazid (H)-resistance from 4.4% (Nationwide Survey into Anti-TB Drug Resistance, 1995–97) to 6%, with an increase in rifampicin (R)-resistance from 1.3% to 1.5%. With respect to MDR-TB, a slight increase was found, from 1.1% at the first survey to 1.4% at the second.12
Resistance to anti-TB drugsResistance to anti-TB drugs is the result of spontaneous mutations in the genome of M. tuberculosis and not the result of horizontal gene transfer.13 The mutations that produce resistance occur at rates that are predictable for each drug, as shown in Table 1. So, for example, the occurrence of a mutant microorganism resistant to H occurs for every 105 or 106 bacilli and a mutant resistant to R for every 107 or 108 bacilli. The mutation of M. tuberculosis occurs independently for each one of the drugs; hence the possibility of the occurrence of associated resistance is equal to the product of their respective resistance rates. Therefore, for a mutant to appear that is naturally and simultaneously resistant to both R and H, theoretically a population of 1012 or 1014 microorganisms would be necessary, making the possibility that it would lodge in the human body highly unlikely.1
Frequency of drug-resistant mutants to anti-TB drugs.a
| Rifampicin | 1 drug-resistant mutant for every 107–8 bacilli |
| Isoniazid | 1 drug-resistant mutant for every 105–6 bacilli |
| Ethambutol | 1 drug-resistant mutant for every 105–6 bacilli |
| Pyrazinamide | 1 drug-resistant mutant for every 102–4 bacilli |
| Streptomycin | 1 drug-resistant mutant for every 105–6 bacilli |
| Ethionamide | 1 drug-resistant mutant for every 103–6 bacilli |
Adapted from Canetti et al. Advances in techniques of testing mycobacterial drug sensitivity, and the use of sensitivity tests in tuberculosis control programmes.85
With selection, particularly as a result of inappropriate treatment management, drug-resistant organisms multiply and become dominant. Once created, drug-resistant strains may be transmitted to individuals who were not previously exposed to anti-TB drugs (primary resistance).14,15 The types of resistance to M. tuberculosis may be summarized as follows:16
- •
Natural resistance: resulting from natural mutation irrespective of previous drug exposure and directly proportional to the number of mutant bacilli;
- •
Primary resistance: cases of resistance in individuals known not to have been previously exposed to anti-TB drugs;
- •
Acquired resistance: cases of resistance in which patients were previously submitted to TB treatment, generally inadequately.
Resistance to H is more often due to a mutation in the katG gene that codifies the katG enzyme, a catalase-peroxidase enzyme. Studies conducted by Zhang et al. at the beginning of the 1990s showed that H is, in fact, a pro-drug that is activated by the katG enzyme.17,18 A later study conducted by Heym et al. identified the katG gene in the chromosome of various mycobacteria including M. tuberculosis and, in addition, these investigators studied the mutations that affect this gene in the isolates of clinical specimens that are H-resistant.19 A great number of reports indicate that mutations in the katG enzyme are responsible for around 60% of cases of isoniazid-resistant strains isolated from patients.19–25 Nevertheless, some mutations in other genes have been identified in isoniazid-resistant strains, including the inhA gene, which codifies the enzyme of mycolic acid involved in the formation of the M. tuberculosis cell wall, and the ahpC gene that codifies alkyl hydroperoxidase, among others.23,26,27 A study carried out in Brazil using isoniazid-resistant strains (n=69) from three states (Rio de Janeiro, São Paulo and Rio Grande do Sul) revealed a predominance of the katG gene mutation, although other mutations (in the inhA and ahpC genes) have also been identified.23
Rifampicin inhibits the beta subunit of the RNA polymerase. The rpoB gene codifies this enzyme and was initially identified in Escherichia coli and Mycobacterium leprae and, later, in M. tuberculosis.28 The rpoB gene mutations are identified in 90–98% of rifampicin-resistant strains. With the advances in molecular biology techniques, the fragment sequencing of the rpoB gene has proven to constitute a rapid way of detecting R-resistance, substituting the slower classic method (the proportion method) that is used routinely in most services.29,30
In recent decades, knowledge on the genetic mechanisms of M. tuberculosis resistance to anti-TB drugs has grown, and this has also facilitated the development of more accurate and faster techniques for detecting resistance.31–40 Nevertheless, the limited availability of laboratories with the capacity to routinely offer these new techniques of diagnosing resistance represents an impediment to their wider use in countries in which the burden of this disease is high.
Diagnosis of resistance to anti-TB drugsLaboratory methods of diagnosing resistance to anti-TB drugs may be classified as phenotypic (based on culture growth in the presence of drugs) or genotypic (i.e. identification of the presence of mutations that confer resistance).
Phenotypic methodsPhenotypic methods, also referred to as drug susceptibility tests, can be performed in solid or liquid media. The susceptibility tests used in solid media cultures (Lowenstein–Jensen and Ogawa–Kudoh) are: (a) the proportion method, (b) the absolute concentration method, and (c) the resistance ratio method. The classic proportion method is the most commonly used both worldwide and in Brazil. One of the limitations of the drug susceptibility tests performed in solid media is the delay, as these tests generally require around two months for results to be released.
Liquid medium, using the BACTEC MGIT 960 system, significantly reduces the time involved in detecting M. tuberculosis to two weeks and after 1–2 weeks the results of the susceptibility test are available.2 The sensitivity and specificity of the MGIT 960 system (96% and 94.6%, respectively) are similar to those found with the proportion method, and this technique has already been validated for use in Brazil.41 The greatest disadvantage of the liquid medium technique is its high cost.2 The accuracy of susceptibility testing varies in accordance with the drug tested, accuracy being greater in the case of rifampicin and isoniazid and poorer in the case of ethambutol and streptomycin.2
Genotypic methodsThe genotypic methods, also referred to as molecular methods, are based on the detection of the mutations that confer resistance to anti-TB drugs. Many genotypic methods have been developed over recent years in response to the urgent need to be able to detect resistance to anti-TB drugs rapidly. The molecular tests most commonly used worldwide are the GenoType MTBDRplus, the INNO-LiPA Rif.TB, and the Xpert MTB/RIF assays. The GenoType MTBDRplus assay (Hain Lifescience) detects mutations in the katG, inhA and rpoB genes, identifying R- and H-resistance. The sensitivity and specificity of the method for the detection of R-resistance are 98% and 99%, respectively, while for the detection of H-resistance, sensitivity is 85% and specificity 99%.42 The INNO-LiPA Rif.TB assay (sensitivity 97%, specificity 99%) detects R-resistance by detecting the rpoB gene mutation.42 Finally, the Xpert MTB/RIF assay identifies R-resistance, with results being available in around two hours and without any need for a sophisticated laboratory infrastructure, allowing the test to be performed at the site where the patient is receiving care. The reported sensitivity for a diagnosis of TB using the Xpert MTB/RIF assay varies according to the study, ranging from 70% to 100% in cases of positive culture and around 60% in cases of TB in which culture is negative. The specificity of the method ranges from 91% to 100%. For the detection of R-resistance, sensitivity and specificity are 98% and 99%, respectively.32–40,43
Despite the promising perspective of the use of molecular tests, also known as rapid molecular testing, for the diagnosis of resistance, the WHO has warned that these tests need to be validated in the countries in which they are to be used, since the frequency of the mutations may vary in accordance with the region.32 In Brazil, validation studies of these new rapid tests for the diagnosis of resistance are currently being conducted and may constitute an important step toward their future use in this country.
In Brazil, the classic proportion method has been the technique most commonly used in the healthcare network; however, the new methods are already available in some reference centers. Increasing access to these new methods for the rapid detection of resistance in routine conditions should constitute an important challenge for the National Program for the Control of Tuberculosis (PNCT).
The treatment of MDR-TBMDR-TB is both costly and complex to treat, since second-line drugs are required that are associated with a higher incidence of adverse reactions.44,45 In addition, treatment is more prolonged compared to when first-line drug regimens are used. The accumulated evidence of MDR-TB treatment outcome remains tenuous and is based on observational studies and expert opinions. The lack of uniformity with respect to the drugs and regimens used (duration of treatment, supervised administration versus self-administration), represents an important obstacle when comparing the different studies. Nevertheless, some publications have indicated that the organization of the healthcare services and the use of supervised treatment are factors that contribute toward increasing success rates.46–48 In addition, mathematical models have suggested that this treatment may be cost-effective, even in countries with limited financial resources.49
The control of resistant TB, particularly in cases of MDR-TB and XDR-TB, represents an important challenge that has to be confronted. Efforts must be made to improve management of these cases, focusing particularly on identifying new drugs that would permit shorter, more effective treatment regimens to be implemented in cases of MDR-TB and XDR-TB.50,51
The ideal treatment for MDR-TB, defined as TB that is simultaneously R- and H-resistant (associated or not with resistance to other first-line drugs), remains a challenge that has to be confronted within the context of current knowledge. The principal difficulties include: the high cost of treatment, its relatively poor efficacy, the side effects of the available drugs and the prolonged duration of treatment. A recent meta-analysis based on retrospective cohort studies on MDR-TB treatment outcome showed a mean success rate of 69% and, furthermore, the superiority of supervised treatment regimens of at least 18 months’ duration.52
Some basic principles must be observed when planning an adequate treatment regimen for MDR-TB: (1) the association of at least four drugs proven to have antimycobacterial efficacy, three of which should not have previously been used by the patient; (2) use of a fluoroquinolone with anti-TB activity (ofloxacin, levofloxacin or moxifloxacin); (3) use of an injectable drug (streptomycin, amikacin, kanamycin or capreomycin); and (4) prolonged, supervised treatment (18–24 months) carried out in a tertiary referral unit.53
Prior to 1995 in Brazil, some experiences were initiated in reference centers in São Paulo, Rio de Janeiro and Porto Alegre in an attempt to elaborate MDR-TB treatment regimens, and between 1995 and 1998 a multicenter protocol was implemented.54 In 2000, the Brazilian Ministry of Health considered the standardized 18-month, 5-drug regimen (amikacin, clofazimine, terizidone, ethambutol and ofloxacin) as validated and adopted it for use in this country. The MDR-TB epidemiological surveillance system was also implemented in that year, with further improvements being included in 2004.
The modifications introduced by the Brazilian Ministry of Health's National Program for the Control of Tuberculosis (PNCT) in 2009 with respect to the treatment of MDR-TB were: (a) to substitute the fluoroquinolone ofloxacin for high-dose levofloxacin; and (b) to promote the use of streptomycin (S) rather than amikacin (AM), although the use of AM is still recommended in two situations: (1) for patients who have already used S in previous treatments; and (2) in cases of proven in vitro resistance to streptomycin.55 In relation to the fluoroquinolone, the decision was made to use high-dose levofloxacin for two reasons: (a) few studies had been conducted on the prolonged use of moxifloxacin and (b) there was evidence suggesting this drug as a future alternative for reducing the duration of treatment in treatment-naïve patients.56
Therefore, the treatment regimen currently used for MDR-TB in Brazil consists of five drugs in the intensive phase (streptomycin, ethambutol, levofloxacin, pyrazinamide and terizidone) and three in the maintenance phase (ethambutol, levofloxacin and terizidone), as described in Table 2. Streptomycin or amikacin is used for five days a week in the first two months and then three times a week for the next four months.
Treatment regimens for multidrug-resistant tuberculosis (MDR-TB).
| Regimen | Drug | Doses per weight range | Months | |||
|---|---|---|---|---|---|---|
| ≤20kg | 21–35kg | 36–50kg | >50kg | |||
| 2S5ELTZIntensive phase1st stage | Streptomycin | 20mg/kg | 500mg/day | 750–1000mg/day | 1000mg/day | 2 |
| Ethambutol | 25mg/kg | 400–800mg/day | 800–1200mg/day | 1200mg/day | ||
| Levofloxacin | 10mg/kg | 250–500mg/day | 500–750mg/day | 750–1000mg/day | ||
| Pyrazinamide | 35mg/kg | 1000mg/day | 1500mg/day | 1500mg/day | ||
| Terizidone | 20mg/kg | 500mg/day | 750mg/day | 750mg/day | ||
| 4S3ELTZIntensive phase2nd stage | Streptomycin | 20mg/kg | 500mg/day | 750–1000mg/kg | 1000mg/day | 4 |
| Ethambutol | 25mg/kg | 400–800mg/day | 800–1200mg/day | 1200mg/day | ||
| Levofloxacin | 10mg/kg | 250–500mg/day | 500–750mg/day | 750–1000mg/day | ||
| Pyrazinamide | 35mg/kg | 1000mg/day | 1500mg/day | 1500mg/day | ||
| Terizidone | 20mg/kg | 500mg/day | 750mg/day | 750mg/day | ||
| 12ELTMaintenance phase | Ethambutol | 25mg/kg | 400–800mg/day | 400–800mg/day | 1200mg/day | 12 |
| Levofloxacin | 10mg/kg | 250–500mg/day | 500–750mg/day | 750–1000mg/day | ||
| Terizidone | 20mg/kg | 500mg/day | 750mg/day | 750mg/day | ||
The number preceding the abbreviation indicates the number of months of treatment. The subscript number after the letter indicates the number of days per week on which the drug has to be administered. S, streptomycin; E, ethambutol; L, levofloxacin; Z, pyrazinamide; T, terizidone.
The duration of the MDR-TB treatment regimen is 18–24 months and treatment administration should be supervised in the reference unit where the patient is being treated or in the basic healthcare unit closest to the patient's home (shared supervision).55
According to the MDR-TB data system, 6136 cases were notified in Brazil between January 1992 and June 2012. The cohort analysis of the treatment outcomes of patients entering the system between January 1992 and December 2010 showed a mean success rate of 60.6%, although there were regional differences between states attributed to factors such as the organization of the healthcare service, the presence of comorbidities and a delay in detecting cases of resistance.57
Treatment of extensively drug-resistant tuberculosis (XDR-TB)Extensively drug-resistant tuberculosis (XDR-TB), defined as TB that is resistant to R, H, a fluoroquinolone and a second-line injectable drug (amikacin, kanamycin or capreomycin), represents a serious public health issue in some regions of the world today. Studies evaluating the treatment of XDR-TB have, in general, shown disappointing results, with poor success rates,58,59 although a study conducted in Peru with a small number of patients reported a cure rate of 60%.60 A recent, retrospective cohort study (n=174) conducted in South Africa in a population with an elevated TB/HIV co-infection rate, showed an early mortality rate of 32% and identified the following independent factors as being responsible for reducing the number of deaths: the use of moxifloxacin in the therapeutic scheme and a regimen containing a greater number of drugs. On the other hand, the presence of a pretreatment culture with proven multidrug-resistance was found to be a factor indicative of poorer prognosis.61 More recent studies have indicated the importance of linezolid, an oxazolidinone, in association with other anti-TB drugs in individualized regimens. Nevertheless, the high cost of this drug and the development of severe adverse effects such as myelosuppression and peripheral neuropathy constitute important obstacles to its use in the majority of tertiary TB management centers.62–70
In summary, there is still no consensus on the most appropriate treatment regimen for XDR-TB. Ongoing research studies with new drugs may show promise in the future and may permit more rational treatment regimens for these patients.50,51 Cases of XDR-TB should be monitored in a tertiary reference center, with individualized treatment regimens consisting of combinations of reserve drugs.9
Adjuvant surgical treatment of MDR-TB and XDR-TBParticularly in MDR-TB, a negative sputum smear or a significant reduction in the bacillary load is desirable prior to surgery in order to minimize the incidence of recurrences, bronchopleural fistula and postoperative empyema. The principal indications for the adjuvant surgical treatment of MDR-TB are: (a) persistently positive sputum despite optimized treatment; (b) localized disease, cavitary pulmonary tuberculosis with a high risk of recurrence and cavities with no signs of regression during treatment, and in cases of unilateral lung destruction; (c) profile of resistance to at least four drugs; (d) multiple recurrences; (e) repeated hemoptysis and/or secondary infections.71–78
The presence of a cavitary lesion reinforces the need for early surgery in view of the difficulty of drug penetration and the greater population of bacilli. The drugs should be maintained for a prolonged period following surgery (18–24 months). The most recent studies conducted with a more careful selection of patients show better results with respect to mortality rates, complications and recurrences.74–76
Although few studies have been published, the indications of surgery as adjuvant therapy in cases of XDR-TB are similar to those for MDR-TB: patients with a localized lesion and lack of an initial response to treatment.75,76,78
New drugs for the treatment of TBThe development and validation of new drugs for the treatment of TB are necessary to allow more appropriate treatment regimens to be elaborated, focusing on the following objectives: (a) shortening the treatment time in cases of TB and of latent TB infection (LTBI); (b) reducing drug interaction with antiretroviral drugs; and c) identifying more effective and safer therapeutic options for MDR-TB and XDR-TB.55
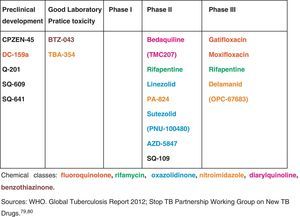
Some new drugs and others already established on the market for other indications are currently being evaluated in clinical trials for TB treatment. Fig. 1 shows some of the drugs that appear promising for future use in TB.79,81 At present, two new drugs are showing results promising in clinical trials for treatment of MDR-TB: bedaquiline (TMC 207) and delamanid (OPC 67683). In December 2012, bedaquiline, a diarylquinoline with a novel mode of action specifically inhibiting mycobacterial ATP synthase, received approval from the Food and Drug Administration (FDA) for the treatment of pulmonary MDR-TB as part of combination therapy in adults.82,83 In its turn, delamanid, a new nitroimidazole derivative, that inhibits mycolic acid synthesis, was associated with an increase in sputum conversion at 2 months among patients with MDR-TB (45.4% as compared with 29.6% of patients who received a background drug regimen plus placebo).84
The development pipeline for new TB drugs.
In view of the epidemiological context of tuberculosis, particularly in countries with a high burden of the disease, the use of more rapid methods for diagnosing the disease and molecular resistance tests, particularly R- and H-resistance, is urgently required. Furthermore, new drugs need to be developed for the treatment of MDR-TB and XDR-TB.
Conflict of interestThe authors have no conflict of interest to declare.