Antiretroviral therapy use has led to a decline in HIV-related mortality yet disparities by gender and/or sexual orientation may exist. In this study, we estimated hazards of death in people living with HIV (PLWH) according to gender and sexual orientation.
MethodsWe included PLWH ≥ 18 years enrolled between 2000 and 2018 at INI/Fiocruz, Rio de Janeiro, Brazil. Participants were grouped as cisgender or transgender women, cisgender men who have sex with men (MSM) or men who have sex with women, or cisgender men with unknown sexual orientation. We assessed disparities in the hazard of death using Cox proportional hazards models.
ResultsAmong 5,576 PLWH, median age at enrollment was 35 years, 39% were MSM, 28% cisgender women, 23% men who have sex with women, 5% transgender women, and 5% men with unknown sexual orientation. A total of 795 deaths occurred in 39,141 person-years of follow-up. Mortality rates per 1,000 person-years were: 82.4 for men with unknown sexual orientation, 24.5 for men who have sex with women, 18.3 for cisgender, 16.6 for transgender women, and 15.1 for MSM. Compared to MSM, men with unknown sexual orientation had the highest death hazard ratio (adjusted hazard ratio [aHR] 2.93, 95% confidence interval [CI] 2.35–3.81), followed by men who have sex with women (aHR 1.17, 95%CI 0.96, 1.43); death hazard ratios for cisgender and transgender women were not statistically different.
ConclusionWe observed disparities in the hazard of death for men with unknown sexual orientation and men who have sex with women despite universal access to antiretroviral therapy in Brazil. Future work should characterize and assist men with unknown sexual orientation with tailored policies and interventions. Increased hazard of death was not observed for transgender women, which probably results from interventions implemented in our service to reach, engage, retain, and support this population.
Scale-up of antiretroviral therapy (ART) has led to increased survival of people living with HIV (PLWH). As result, life expectancy of PLWH in high income countries approaches that of the population not living with HIV [1]. A study using data from multiple clinical cohorts in Latin America and the Caribbean including over 30,000 participants estimated that life expectancy at age 20 years among PLWH increased from 31.0 years in 2003–2008 to 69.5 years in 2013–2017 [2]. Moreover, PLWH who initiated ART with CD4 counts greater than 200 cells/mm³ had similar life expectancy as the population not living with HIV [2], highlighting the continued need for early HIV diagnosis and ART therapy initiation to fully realize the life expectancy gains.
Nonetheless, disparities in mortality rates and survival among PLWH stratified by demographic characteristics such as sex assigned at birth, gender and sexual orientation exist. Several observational studies found that cisgender women had lower mortality rates and longer life expectancy than cisgender men [2,3], although mortality rates among cisgender women vary largely across several regions [4]. A recent Dutch study reported that transgender women had higher mortality risk than cisgender women (standardized mortality ratio 2.8, 95% confidence interval [CI] 2.5–3.1), and cisgender men (standardized mortality ratio SMR 1.8, 95% CI 1.6–2.0) [5]. Additionally, heterosexual cisgender men have lower life expectancy than cisgender women and gay, bisexual and other cisgender men who have sex with men (MSM) in Latin America [2].
These disparities may possibly be explained by social determinants of health (i.e., income level/poverty, employment, education, social support, discrimination, and stigma) interdependently with late presentation to care. Social determinants of health are the root of health inequalities, hindering access to health care and impacting all steps of the HIV care continuum, including HIV testing, early diagnosis, ART initiation, virologic suppression, and retention to care [6–9]. Moreover, the burden of social determinants varies across gender and sexual orientation groups [10–12], with sexual and gender minorities being additionally affected by minority stress (i.e., stigma, prejudice and discrimination against their identities), thus increasing their risk for negative health outcomes [13].
Brazil has made steady progress towards achieving UNAIDS 95-95-95 targets by 2030 [14], and in 2020, 88% of PLWH were diagnosed, 71% were receiving ART, and 63% were virally suppressed [15]. However, late diagnosis prevails and in 2016, 44% of PLWH in Brazil initiated ART with CD4 counts below 350 cells/mm3[6]. Furthermore, two Brazilian studies have shown disparities in late presentation (i.e., starting ART with CD4 < 350 cells/mm3 or with AIDS defining illness) by sex, gender, and sexual orientation. Rodrigues et al. found that, compared to cisgender women, MSM and heterosexual cisgender men had higher odds for late presentation (Rio de Janeiro, Brazil, 2005–2018) [8], while Pacheco et al. found that compared to MSM, cisgender women, heterosexual cisgender men, and men with unknown sexual orientation had higher odds for late presentation (Goiania, Brazil, 2009–2012) [16].
In this study, we estimated mortality rates and survival curves among PLWH over the past 20 years and investigated possible disparities in the hazard of death by gender and sexual orientation while controlling for confounders.
MethodsStudy design and participantsThe Instituto Nacional de Infectologia Evandro Chagas, Fundação Oswado Cruz (INI/Fiocruz) is a reference institution for care, research, and training in infectious diseases in Rio de Janeiro, Brazil, established in 1918. A longitudinal observational clinical cohort maintains data on individuals receiving HIV care since 1986, which has been used in several studies [17,18]. Data on demographic variables, ART use, laboratory results (including CD4 count and HIV viral load [VL]) and clinical diagnosis (opportunistic illnesses, hospitalizations, chronic diseases) are collected onto standardized forms and updated regularly with outpatient and inpatient documentation. Collected data are routinely reviewed through internal and external procedures for accuracy. For this study, we included adults living with HIV (PLWH, aged ≥ 18 years at cohort entry) enrolled from January 1, 2000, to December 31, 2018 and who had a minimum follow-up of one day. This procedure ensures that the study population included in this survival analysis is in fact at risk (alive at time zero) of having the study event of interest (death) during follow-up.
Main exposure of interestStudy participants were categorized according to assigned sex at birth, self-reported gender and sexual orientation as cisgender women, transgender women, cisgender men who have sex with women (MSWo), gay, bisexual and other cisgender men who have sex with men (MSM) and cisgender men with unknown/not disclosed sexual orientation (Munk). One transgender man was excluded from the study.
Outcome and follow-upThe primary outcome was death by any cause during follow-up. Individual's follow-up time was calculated for each participant by subtracting the date of the end of follow-up from the date of the start of follow-up. Start of follow-up was defined as the date of cohort entry, here defined by the date of the first medical appointment at INI/Fiocruz. End of follow-up was defined as date of death, date of last clinic or laboratory visit or date of study closure (December 31, 2018), whichever occurred first. To account for Brazilian HIV care recommendations of clinic visits every six months [19], we added 180 days to the date of last clinic or laboratory visit prior to defining the end of follow-up. The assumption being that participants are deemed lost on the date that they were expected to return to the clinic. We explored the impact of this assumption in a sensitivity analysis, described below, and the results are presented in the Supplementary Material. Accurately describing mortality rates and hazards of death requires robust death ascertainment. Particularly, if individuals deemed lost to follow-up are in fact deceased, this could lead to potential bias in the estimated survival. To improve death ascertainment and reduce the possibility of this bias, information from PLWH in cohort are routinely linked with the State of Rio de Janeiro death registry to recover potential information regarding death (SIM – Sistema de Informação de Mortalidade). This linkage uses a previously validated algorithm published elsewhere [20].
Status at the end of an individual's follow up was categorized into three mutually exclusive groups: a) Dead: for those with death date before December 31, 2018; b) Alive: for those not known to have died before December 31, 2018 and with end of follow up occurring after January 1, 2018; and c) Loss to follow-up (LTFU): for those not know to have died before December 31, 2018 and with end of follow up before January 1, 2018.
Given the extensive effort to comprehensively determine vital status, we performed a sensitivity analysis that considered the censoring date for all non-deceased participants as December 31, 2018, the study closure date (see Supplementary Material).
CovariatesSociodemographic variables at cohort entry included age, self-reported race/skin color (White, Pardo/mixed, Black, Other), education (< or ≥ 9 years [which is equivalent to have completed primary education in Brazil]), emigrated to Rio de Janeiro (yes/no). Laboratory variables included: CD4 and VL at enrollment (closest result to cohort entry, +/- 365 days) and CD4 nadir (lowest CD4 value ever recorded). Clinical variables included: lifetime injection drug use (IDU, yes/no), hepatitis B infection (defined by the presence of HBsAg) and hepatitis C infection (defined by the presence of hepatitis C antibodies) diagnosed at any time before or during follow-up, AIDS defining illness at enrollment (one year prior to 30 days after enrollment), AIDS defining illness during follow-up (from 30 days after enrollment to 30 days before end of follow-up), hospital admission during follow-up (due to any cause; we excluded hospital admissions that ended in death), ART use (yes/no, defined as use of at least two nucleoside reverse transcriptase inhibitors with a protease inhibitor, a non-nucleoside reverse transcriptase inhibitor, or an integrase inhibitor for at least 30 days before end of follow-up), years of ART use (calculated from the start of first ART regimen to date of death or censoring), and year of cohort entry. For the analyses, AIDS defining illnesses were categorized as: tuberculosis, non-tuberculosis opportunistic infections (OI), and AIDS malignancies.
Statistical analysisDescriptive statistics for sociodemographic, laboratory, and clinical variables were compared using Kruskal-Wallis or t-test for continuous variables and chi-square tests for categorical variables. All-cause mortality rates per 1000 person-years (PY) and 95% confidence intervals (CI) were calculated overall and by gender/sexual orientation using Poisson regression models with robust standard errors. Cox proportional hazards regression models were used to assess whether gender/sexual orientation groups were associated with mortality. All covariates listed above were tested in unadjusted Cox regression models. Covariates with p-value < 0.20 were included in the initial adjusted Cox regression model. The final adjusted model included gender/sexual orientation and other covariates with p-value <0.05. Age, square root transformed CD4, log10 transformed VL, years of ART use and year of cohort entry were included in the models using cubic splines with 3-knots to relax the assumption of linear association. Missing data (i.e., CD4 and VL at enrollment and nadir CD4 and education) were multiply imputed using predictive mean matching and 10 imputation replications. Imputation models included all covariates in the initial model as well as the outcome of interest. Participants with race/skin color categorized as “other” were removed from the regression models, due to small sample size (n = 13). Analyses were performed in R version 4.1.2, using “survival”, “mice”, “rms”, “ggplot” packages.
EthicsThis study was approved by INI/Fiocruz institutional reviewer board (CAAE: # 88392618.4.0000.5262) and was conducted according to the Declaration of Helsinki principles. Participants provided written informed consent.
Role of the funding sourceThe funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All the authors had full access to the data in the study and had final responsibility for the decision to submit it for publication.
ResultsStudy participants characteristicsAmong 5576 participants included in this study, 28.2% were cisgender women, 5.3% transgender women, 38.7% MSM, 22.7% MSWo and 4.9% Munk. At enrollment, overall median age was 35 years (IQR 28–43), with transgender women being the youngest group (median age 31 years, IQR 26–38) and Munk the oldest group (median age 38 years, IQR 28–47). Most of the participants were Pardo/mixed race (34.6%) or Black (20.6%), with the largest proportion of Black among cisgender and transgender women (25.5% and 25.4%, respectively) and the lowest among Munk (19.2%). Forty-six percent of the participants had less than nine years of education, and the highest proportion of low education was observed among cisgender women (60.9%) and the lowest among MSM (25.6%). A quarter of the study population had emigrated to Rio de Janeiro (not different across all gender/sexual orientation groups). Overall, less than 1% of the study population reported lifetime IDU (Table 1).
Study population characteristics by gender and sexual orientation, INI-Fiocruz, Rio de Janeiro, Brazil (2000–2018).
MSM, gay, bisexual and other cisgender men who have sex with men; MSWo, cisgender men who have sex with women; Munk, cisgender men with unknown/not disclosed sexual orientation; IQR, interquartile range; IDU, injection drug use; ART, antiretroviral therapy; OI, opportunistic illness; VL, viral load.
At enrollment, the median CD4 was 302 cells/mm³ (IQR 111–535), lowest among MSWo (median 208 cells/mm³, IQR 69–414) and highest among transgender women (median 485 cells/mm³, IQR 252,734). The median nadir CD4 was 197 cells/mm³ (IQR 62–347), also lowest among MSWo (median 123 cells/mm³, IQR 35–264) and highest among transgender women (median 327 cells/mm³, IQR 162–587). Overall, 15.3% of the participants had tuberculosis at enrollment, with the highest frequency observed among MSWo (24.7%) and lowest among MSM (11.7%). Non-tuberculosis OIs at enrollment were observed in 15% of the study population; most frequent among Munk (24.3%) and least among transgender women (2.7%). AIDS malignancies were present in 3.3% of the study population at enrollment, with the highest frequency among Munk (6.2%), followed by MSM (5%). Overall prevalence of Hepatitis B was 5.2%, highest among transgender women (8.7%) and lowest among cisgender women (3.1%). Hepatitis C prevalence was 6.8% overall, not different across gender/sexual orientation groups (Table 1).
Overall, 83% of the participants used ART for at least 30 days, with a median duration of 5.4 years (IQR 1.3–9.5). The lowest frequency of ART use and the shortest ART duration were observed among Munk (65.8% and median 2.3 years, respectively). During follow-up, 7.7% of the participants had tuberculosis (most frequent among MSWo, 10.6%); 12.8% had non-tuberculosis OIs (most frequent among cisgender women, 15.5%); and 1.8% had AIDS malignancies (most frequent among Munk, 3.7%) (Table 1).
Mortality rates and gender/sexual orientation disparitiesMedian follow-up time was 6.5 years (IQR 2.8–10.6), longest among cisgender women (median 7.8 years, IQR 4.1–11.5) and shortest among Munk (median 2.9 years, IQR 0.8–5.9). A total of 795 deaths were observed, yielding a mortality rate of 20.3/1000PY (95% CI 18.9–21.8). The highest mortality rate was observed among Munk (82.4/1000PY, 95% CI 66.4–100.9) and the lowest among MSM (15.1/1000PY, 95% CI 13.3–17.2). A total of 468 participants were deemed LTFU, yielding a rate of 12.0/1000PY (95% CI 10.9–13.1), with the highest among Munk (47.8 /1000PY, 95% CI 35.8–62.1) and lowest among cisgender women (8.4/1000PY, 95% CI 6.9–10.1) (Table 2).
Mortality overall and by gender/sexual orientation, INI-Fiocruz, Rio de Janeiro, Brazil (2000–2018).
MSM, gay, bisexual and other cisgender men who have sex with men; MSWo, cisgender men who have sex with women; Munk, cisgender men with unknown/not disclosed sexual orientation; PY, person-years; IQR, interquartile range; LFTU, loss to follow-up; CI, confidence interval.
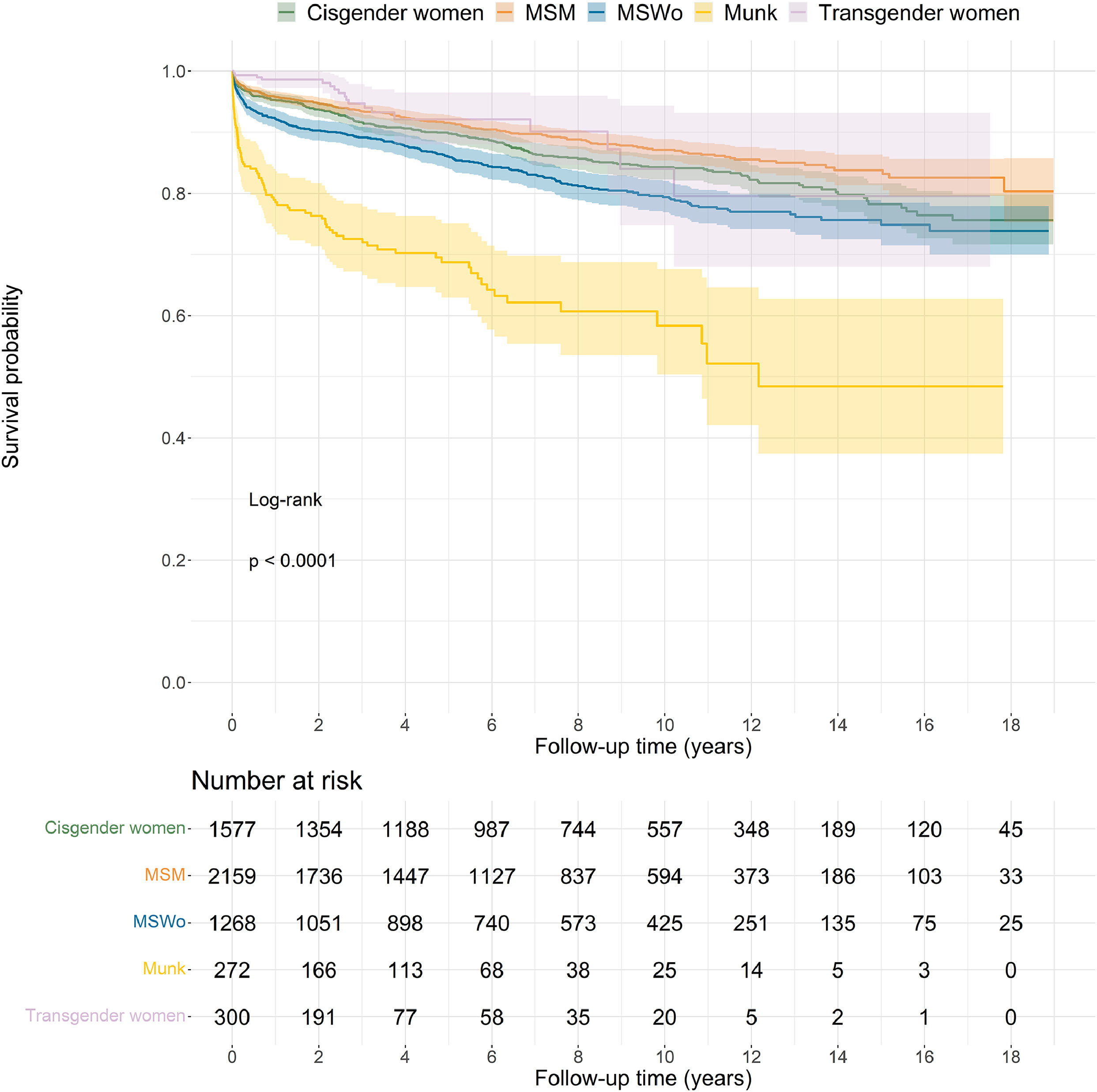
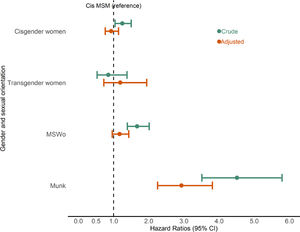
Gender and sexual orientation groups differed significantly in survival (log-rank p-value < 0.0001; Fig. 1). The survival experience for Munk deviates significantly from the other groups and is marked by an initial steep drop in survival. By the end of the first year, survival probability was 78% for Munk whereas it was 92% for MSWo, 96% for MSM and 98% for cisgender and transgender women.
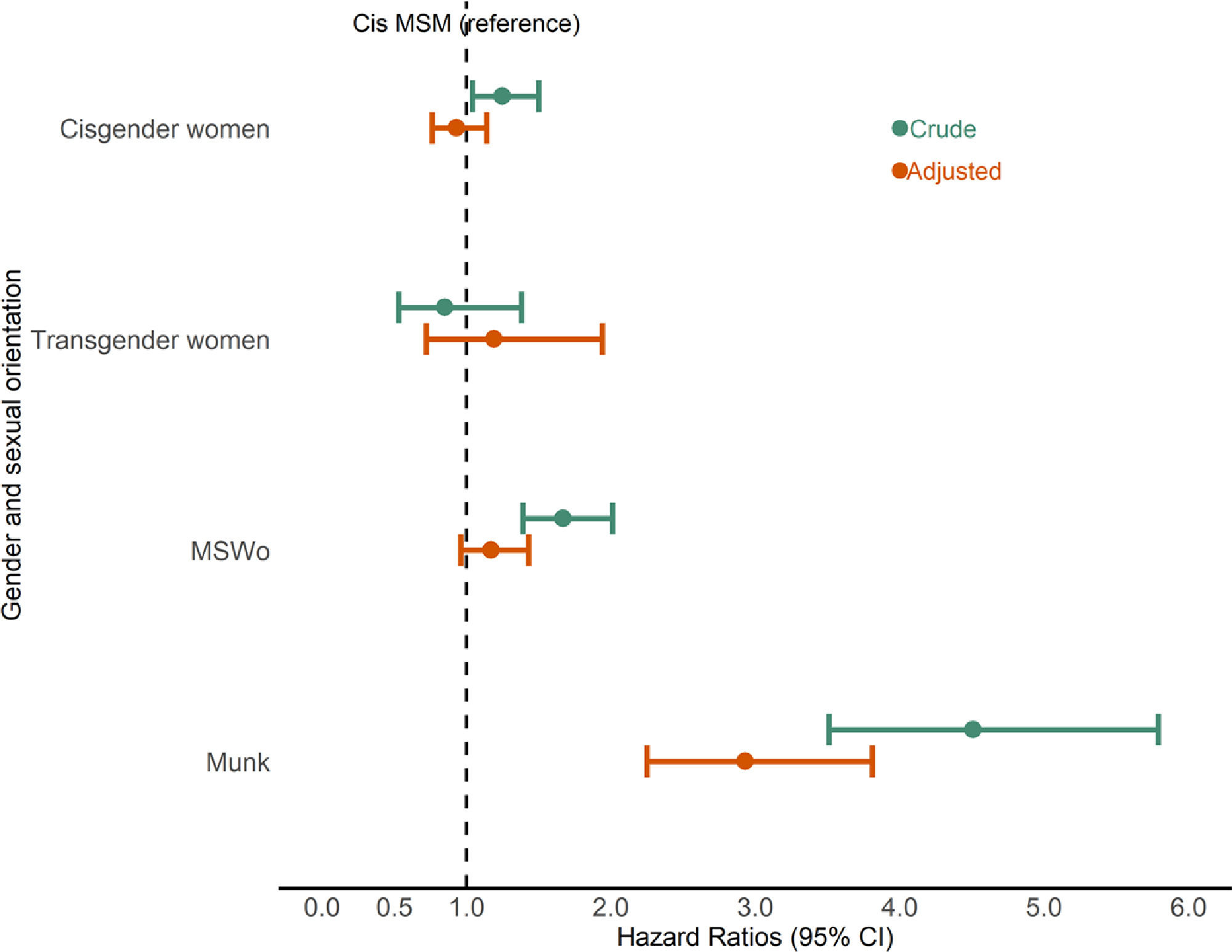
Compared to MSM, the crude hazard of death was significantly higher for Munk (crude hazard ratio [cHR] 4.51, 95% CI 3.51 – 5.79), MSWo (cHR 1.67, 95% CI 1.39–2.01) and cisgender women (cHR 1.25, 95% CI 1.04–1.50); and non- significantly lower for transgender women (cHR 0.85, 95% CI 0.53–1.21) (Fig. 2).
In the final regression model, the adjusted hazard of death remained significantly higher for Munk (adjusted [a]HR 2.93, 95% CI 2.35–3.81) and borderline higher for MSWo (aHR 1.17, 95% CI 0.96–1.43); while for cisgender women (aHR 0.93, 95% CI 0.76–1.14) and transgender women (aHR 1.19, 95% CI 0.72–1.94) the adjusted hazard of death was not significantly different from MSM (Fig. 2).
Furthermore, results of the sensitivity analysis that assumed study closure (December 31, 2018) as the censoring date for all non-deceased participants corroborated the findings described above (Supplementary Material Table 1 and Figs. 1 and 2).
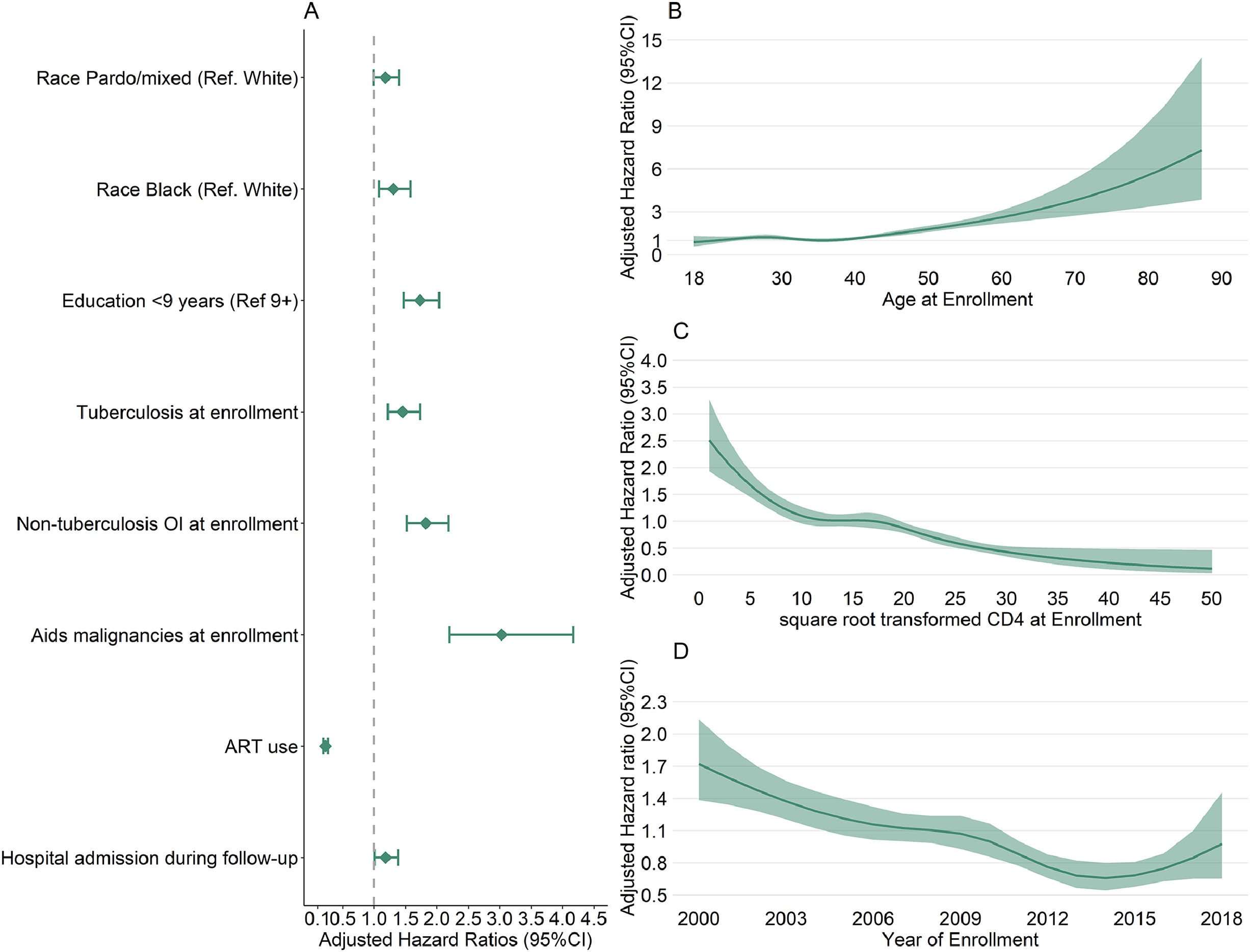
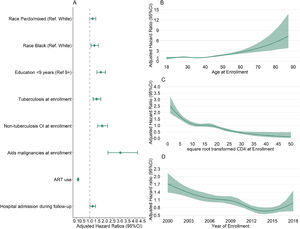
Other factors associated with mortality riskIn the final regression model, Pardo/mixed race and Black races and low education (< 9 years) were factors associated with an increased hazard of death. Tuberculosis, non-tuberculosis OI and AIDS malignancies at enrollment and hospital admission during follow-up were also associated with an increased hazard of death, while ART use decreased the hazard of death. A positive association was observed between increased age and hazard of death, while a negative association was observed between increased CD4 at enrollment and hazard of death. A “W” shape association was observed between year of enrollment and hazard of death, with the hazard of death decreasing from 2000 up to 2006, stabilizing between 2006 and 2010, decreasing between 2010 and 2013 and increasing after 2014 (Fig. 3).
DiscussionIn this study, we found significant disparities in mortality among PLWH according to gender and sexual orientation, with Munk and MSWo having higher hazards of death than MSM. Munk had 3-fold higher hazard of death than MSM after controlling for sociodemographic and clinical confounders, suggesting that other unmeasured variables, such as socioeconomic status (i.e., poverty, employment, housing) and stigma might play a role on the association between sexual orientation and mortality among PLWH. Munk although accounting for less than 5% of our study population represented 11% of the deaths, and had the highest mortality rate, which was 4.5 times higher than MSM. Remarkably, one third of Munk died after cohort entry and their poor survival experience is most pronounced during the first year of follow-up. Munk were older at cohort entry, had the highest proportion of non-tuberculosis OIs and AIDS malignancies diagnosis at enrollment and during follow-up; conversely, they had the lowest proportion of ART use. Though we do not know which sexual orientation would be most applicable to the men in the Munk category, we hypothesize that this group was heterogenous, consisting of a combination of MSM and MSWo. For instance, the high frequency of AIDS malignancies observed in Munk (even higher than MSM) suggests that part of the group consisted of MSM, since Kaposi Sarcoma (historically the most common AIDS malignancy in our cohort) [21,22] is much more common among MSM than other groups due to human herpes virus-8 sexual transmission [23]. Conversely, the Munk group was older and less educated, characteristics that are more similar to the MSWo participants. The unknown sexual orientation status might constitute missing information resulting from severe health conditions at cohort entry, with consequent rapid progression to early death, thus hindering the capture of a comprehensive medical and social history. Alternatively or concomitantly, it might result from concealment of sexual orientation to health care professionals, possibly driven by stigma and discrimination. The rationales to support both hypotheses are detailed below.
As early mortality was most frequent among Munk, this may partially explain the missing information for sexual orientation. The high proportion of Munk participants with missing CD4 at enrollment, missing CD4 nadir, no ART use prior to end of follow-up supports the hypothesis that for at least a fraction of these participants, advanced disease and early mortality soon after cohort entry might have prevented us to collect the sexual orientation information as well as laboratory data. The second hypothesis, concealment of sexual orientation, might have been driven by stigma and discrimination. Sexual concealment may act as a functional adaptation to hostile structural contexts; in societies where heteronormativity norms prevail (including Brazil), sexual and gender minorities are often victim of stigma, discrimination and violence [24]. In Brazil, a multicity population-based survey of MSM conducted in 2016, found that 65% of the respondents had been discriminated against because of their sexual orientation [25]. Interestingly, a prior survey, conducted in 2009 [26], with a similar rationale to that of the 2016 study, had reported a much lower frequency of discrimination based on sexual orientation (28%), pointing to an increase in societal conservatism in most recent years, coupled with increased awareness of what constitutes discriminatory acts [27]. Fear of disclosure of sexual orientation delays access to care [28] as suggested in a previous study with PLWH in Brazil that showed cisgender men with unknown sexual orientation had 2.5 times the odds for late presentation relative to MSM [16].
In our study, we also found that MSWo had a higher hazard of death compared to MSM. This finding was expected as life expectancy among PLWH is lower in heterosexual men compared to women and MSM in Latin America [2]. Moreover, in a previous analysis of our cohort (2000–2011), hazard of AIDS-related death among heterosexual men was twice higher than that of women [18]. Studies in Brazil have shown that late presentation is frequent among heterosexual men. A study conducted in Salvador in 2010–2011 showed that 79% of heterosexual men had first CD4 count < 350 cell/mm3[29]. Also, heterosexual men had higher odds for late presentation than MSM and women in Goiania (2009–2012) [16]. On the other hand, a recent study with a sample of young conscripts in Brazil, mostly heterosexual men, has found that 85% of the participants had never been tested for HIV and that anticipated HIV stigma was associated with delay in HIV testing [30]. Altogether, these findings can be partially explained by cultural and gender norms that prevail in Brazil, hegemonic masculinity is often associated with men's risky behavior (feelings of invulnerability), which include alcohol and drug use, pleasure seeking, lack of self-care, and denial of health information and services [31–34]. Nonetheless, heterosexual men are often left out of HIV prevention and care policies and programs [31,34].
We found that cisgender women had an adjusted hazard of death similar to that of MSM. This finding is somewhat different from a study with PLWH in Latin America and the Caribbean that found lower mortality rate and higher life expectancy among cisgender women than MSM [2], and different from a previous study of AIDS and non-AIDS mortality in our cohort (2000–2011) [18]. Compared to MSM, cisgender women in our study were more likely to be Black (25% of the women, the highest proportion across the groups), less educated (60% had not completed primary school) and had low CD4 counts (nadir and at enrollment). In addition, they were less likely to use ART, had more OIs (tuberculosis and non-tuberculosis) and were more likely to have a hospital admission during follow-up than MSM. These factors probably contributed to cisgender women's higher mortality rates. Results from the Cox regression models suggest that adjustment for these factors resulted in a hazard of death that was not different from MSM.
Transgender women had the second lowest mortality rate in our study (only after MSM). At first, this finding may be surprising since Brazil leads the global statistics for numbers of death and violence against transgender women [35], and the number of homicides against sexual and gender minorities has been increasing steadily over the years [36]. Transgender women are disproportionately affected by HIV, with an estimated 30% prevalence of HIV infection in Rio de Janeiro in 2016 [37]. Moreover, transgender women face barriers to HIV testing, care and treatment, and stigma and discrimination play an essential role in reducing the access of transgender women to medical visits and HIV testing services [38]. To situate our results within the broader context of high mortality risk among transgender women living with HIV [5] we need to highlight some critical findings. First, 72% of transgender women included in our study enrolled after 2014 as a result of a series of interventions developed and implemented to reach and engage transgender women in HIV prevention and care in our clinic [39,40] (i.e., gender neutral space, transgender staff, community engagement, partnership with stakeholders and non-governmental organizations). A large population-based study aiming to recruit transgender women in Rio de Janeiro was conducted in 2015–2016 and those participants were offered routine HIV prevention, HIV care and endocrinological cross-gender care [37,39]. As a consequence, compared to other groups, transgender women included in our study were younger, had the highest CD4 counts (at enrollment and nadir) and the lowest proportion of non-tuberculosis OI at enrollment. Altogether, our findings suggest that the low mortality observed among transgender women enrolled in our cohort may be partly explained by their relative better health when compared to the other groups. Moreover, our findings suggest that active interventions to reach, test and engage transgender women in care may counteract the deleterious structural factors driving mortality in transgender women living with HIV.
Finally, our results underscore the association between social determinants of health and late presentation to HIV care with the risk of death among PLWH. Pardo/mixed and Black race and low education were strongly associated with a higher death hazard. Moreover, proxies of late presentation (i.e., lower CD4 counts and AIDS defining illnesses) were associated with an increased risk of death, while ART use reduced the risk of death. In addition, a worrisome upward trajectory of the hazard of death in most recent years (between 2015 and 2018) was observed, though this finding was not completely unexpected. A previous study from our cohort (2004–2015) has shown that very early mortality (i.e., deaths occurring up to 90 days after enrollment) remained high over the years, unlike deaths occurring between 90 and 365 days after enrollment [41]. Likewise, in the present study, survival curves, particularly for Munk, show a steep drop in survival probabilities in early follow-up. The spread of the HIV epidemic among vulnerable populations, the persistence of late presentation, and the increased of poverty and social inequalities in Brazil over the past decade [42] likely contributed to this finding.
Our study has limitations. First, 8.4% of our study population were deemed lost to follow-up; however, we periodically update vital status for our cohort participants through linkage with the State of Rio de Janeiro mortality database, allowing us to assume with reasonable confidence that participants classified as lost to follow-up were indeed alive as of December 31, 2018 (further corroborated by the sensitivity analysis shown in the Supplementary Material). Second, we did not differentiate causes of death, preventing us to assess gender and sexual orientation disparities related to specific causes of death (AIDS, non-AIDS, violent deaths). Third, we did not have information on potential confounders, mainly socioeconomic status (i.e., income, employment, housing), behavior factors (alcohol, smoking) and stigma. Fourth, most of the transgender women were recently enrolled in the cohort (from 2014 onwards) and future studies will be needed to better assess mortality trends in upcoming years, particularly for this group. Finally, our results are likely not representative of the entire population of PLWH in Brazil given that our participants represent a convenience sample. In addition, we found higher overall mortality rates (period 2000–2018, 20.3/1000 PY) than a recent study including PLWH in Latin America and the Caribbean (the Caribbean, Central and South America network for HIV epidemiology [CCASAnet], period 2003–2017, 17.9/1000 PY) [2] and a previous study from our clinical cohort (INI-Fiocruz, period 2000–2011, 10.2/1000 PY) [18]. It is very likely that differences in the studies’ specific eligibility criteria explain the different rates. Specifically, the current study population includes individuals who did not use ART and whose follow up was minimal. In contrast, the CCASAnet study restricted the study population to ART users [2]. Likewise, the study population of the prior INI-Fiocruz analysis excluded participants with less than 60 days of follow-up and also excluded IDU, Munk, and individuals with no CD4 count [18]. These criteria potentially excluded population groups with higher hazards of death from the two aforementioned studies.
ConclusionsOur results highlight important disparities in mortality risk by gender and sexual orientation among PLWH, and that Munk and MSWo have increased risk of death compared to MSM. Future studies should aim to better characterize and understand this population of men with concealed sexual orientation and their outcomes, including in-depth qualitative research, to help tailor policies and interventions to improve outcomes in this population. Finally, we did not observe an increased risk of death among transgender women, which may be explained by specific strategies (gender neutral space, transgender staff, community engagement, partnership with stakeholders and non-governmental organizations) implemented in our service to reach, engage, retain, and support transgender women.