Environmental surveillance of water sources is important to monitoring viral hepatitis transmission in clinical settings. This study investigated the circulation of hepatitis A (HAV) and E (HEV) viruses in sewage and clinical samples from Argentina. Between 2016 and 2017, 80 raw sewage samples and 86 clinical samples (stool and serum) from suspected cases of hepatitis A and hepatitis E were obtained. HAV and HEV were tested by both real-time and nested PCR. Positive samples were sequenced for genotype determination and phylogenetic analysis. Overall, HAV was recovered in 39% of sewage samples and 61.1% of clinical samples. HEV was detected in 22.5% of sewage samples and 15.9% of clinical samples. HAV was found more frequently in sewage during the winter and in clinical samples in spring; HEV was more prevalent in sewage during summer and in clinical samples in autumn. All HAV isolates belonged to genotype IA and HEV isolates belonged to genotype 3, the most prevalent genotypes in South America. High prevalence of HAV and HEV in environmental and clinical samples in Mendoza, Argentina was observed. These findings reinforce the importance of environmental surveillance and implementation of health strategies to control the spread of HAV and HEV in developing countries.
Hepatitis A and E viruses (HAV and HEV) cause acute, self-limited infections that may vary in severity from asymptomatic to fulminant hepatic diseases.1-4 HAV and HEV are mainly transmitted by ingestion of contaminated food and water. These viruses are endemic in developing countries being often related to socioeconomic levels and sanitary conditions.5,6
Studies have demonstrated HEV antibody prevalence from 5.1 to 20.0% in different regions of Argentina.7-9 Regarding hepatitis A, prevalence rates ranged from 29.7% in Buenos Aires2 to 81.9% in low-income indigenous populations.9
In 2004, a single dose HAV universal immunization program for 12-month-old children was implemented in Mendoza, Argentina. The program has reached high coverage (> 90%) in 2006, resulting in an accentuated drop (< 80%) in the incidence of HAV infection in Argentina in 2017,5,10,11 despite high endemicity still found in areas of inefficient vaccine coverage.
Few studies have investigated the presence of HAV and HEV in environment in Argentina. HAV prevalence from 16.1 to 20.8% was observed, respectively, in river samples and wastewater from Córdoba province,12 while HEV prevalence from 1.6 to 7.6% have been found in Arias-Arenales River, Salta province and in recreational waters of San Roque Dam, Córdoba.13,14 Raw sewage is an important tool to identify virus excreted in stools and it could be used to identify wild and vaccine-derived strains from different preventable infectious diseases.
Mendoza city is located in Central West Argentina where there is reuse of aqueous cloacal matrices for local agriculture after treatment in sewage treatment plants. In this situation, the monitoring of sewage for viral contamination is crucial before the discharge of effluents into water bodies.8,13
To our knowledge, there is no history of environmental detection of HAV and HEV in Mendoza, Argentina. The aim of this study was to describe the circulation of HAV and HEV in environmental and clinical samples from Mendoza.
MethodsSewage sample collection and virus concentrationMendoza is a province of Argentina with an area of 148,827 km2, and a population of 1,729,660 people, located in a zone of temperate continental climate with a pronounced seasonality. The province has 18 counties and the capital is Mendoza city. The municipal sewage system collects domestic wastewater and street drains into the same system. Then, this combined wastewater undergoes a full treatment cycle at two main sewage treatment plants (TP) named Espejo and Paramillos to be later used for irrigation of local crops.
Between 2016 and 2017, a total of 80 raw sewage samples were collected from two wastewater treatment plants (WWTP) (40 samples from Paramillos and 40 samples from Espejo) in Mendoza city. These WWTP receive sewage volume of 1,000,000 inhabitants (62.4% of total population).
Environmental samples were collected in all seasons. At least three sewage samples per place (WWTP) per season were collected in different months in duplicate (two samples of 2 L). Samples were collected monthly from August (2016) to December (2017).
Virus concentration was made according to previously described.15,16 Before concentration, all samples were spiked with bacteriophage PP7 6.8 log10 genome copies/ μL−1, which was used as an internal process control as described previously.17 The concentration method use negative membrane to filtrate water samples as briefly described above. Samples were previously filtered by an AP20 membrane (Millipore Corporation, Billerica, MA, USA) (retention range 0.8-8 μm). Then, each aliquot was filtered again through negatively charged membranes (0.45 μm porosity and 142 mm diameter) (Millipore Corporation, Billerica, MA, USA) using a vacuum pump, with the intention that the viruses will be adsorbed to it. A concentrated filtrate of 2 mL was obtained by centrifugation using Centriprep Concentrator (Millipore) at 1500xg for 10 min and stored at −20°C until processing.16
Clinical samples collectionBetween 2016 and 2017, a total of 86 serum and stool samples were tested, belonging to 11 individuals with recent HAV infection (anti-HAV IgM positive in serum) and 39 suspected hepatitis cases presenting jaundice or related symptoms without anti-HAV IgM. All individuals were pediatric or adult outpatients and inpatients referred to Central Hospital of Mendoza. All patients presented symptoms of hepatitis with negative results for cytomegalovirus, hepatitis B and C virus, herpes virus, and Epstein-Barr virus.
The ethical procedures have been respected and followed in accordance with the standards of the Research Ethics Committee of the Central Hospital, Mendoza (number 03-04-2014-02).
HAV and HEV detectionRNA was extracted from 140 µl of concentrated environmental or clinical samples using a QIAamp™ Viral RNA Kit (Qiagen, city, Germany) according to the manufacturer's instructions. Real-Time qualitative PCR of HAV (5´NCR) and HEV (ORF 3 region) were performed using primers, probes and reaction conditions previously described.15,18,19 Nested RT-PCR for amplification of VP1/2A region of HAV genome was performed for sequencing purpose as described elsewhere.15 Also, a previously described protocol was applied to amplify partial region of ORF2 of HEV genome corresponding to capsid genes.18 The PCR products were loaded onto a 2% agarose gel, electrophoresed and stained with ethidium bromide to visualize bands (expected length, 247 bp for HAV and 197 bp for HEV).
Sequencing and phylogenetic analysesAmplicons were purified using the QIAquick Gel extraction kit (Qiagen) according to the manufacturer's recommendations. Direct nucleotide sequencing reaction was performed in both directions of HAV and HEV cDNA positive samples with a Big Dye Terminator kit (Applied Biosystems, Foster City, CA, USA) and an automatic DNA sequencer (ABI Prism 310; Applied Biosystems).
Multiple alignments were initially performed with the Clustal X program.20 Phylogenetic analysis was performed by using the Maximum Likelihood method under General Time Reversible model as the best-fit nucleotide substitution model. The reliability was assessed by bootstrap resampling of 1000 replicates. These methods were implemented in MEGA 7.0 program.21 Genetic distances were calculated based on nucleotide ‘p’ distances in MEGA 7.0 program, with bootstrap resampling of 1000 replicates. Similarity analysis was performed using the basic local alignment search tool (BLAST - https://blast.ncbi.nlm.nih.gov/Blast.cgi), available online.
ResultsHAV and HEV detection in environmental samplesFrom 2016 to 2017, 80 wastewater samples were collected, 40 from Espejo and 40 from Paramillo. Environmental samples in which HAV or HEV were detected by either methodologies used (qualitative PCR or RT-qPCR) were considered positive. Overall, HAV was detected in 39% of sewage samples (31/80), 12 from Espejo and in 19 from Paramillo. HEV was detected in 22.5% of sewage samples (18/80), 14 from Espejo and 4 from Paramillo. Using nested RT-PCR, HAV and HEV were detected in 20 (25%) and 4 (5%) samples, respectively. Using real-time PCR detection, HAV and HEV were detected in 11 (13.7%) and 14 (17.5%) samples, respectively. HAV and HEV were detected by both methods in six and three samples, respectively. HAV simultaneous detection occurred in all seasons (1 Paramillo sample in Summer and other in autumn; two samples in winter and two samples in spring in each WWTP). The internal process control (PP7 bacteriophage) was detected in 100% of sewage samples (n = 80).
Season distribution of HAV and HEV in environmental samples is shown in Table 1. HAV was detected in all seasons, in 30% (n = 12/40) and 47.5% (n = 19/40) samples from Espejo and Paramillo, respectively. HEV was also found in all seasons, in 35.0% (n = 14/40) and 10.0% (n = 4/40) from Espejo and Paramillo, respectively. As shown in Table 1, HAV was more frequent in Paramillo, while HEV was more frequently detected in Espejo. Highest HAV detection was found during winter while HEV was observed more frequently in summer. HAV was detected at higher rates in all four seasons compared to HEV. No differences in pH related to the detection of HAV and HEV in the different seasons were observed (Table 1).
HAV and HEV detection in sewage samples from Mendoza, Argentina.
Between 2016 and 2017, a total of seven fecal samples and 10 serum samples were collected from 11 HAV suspected individuals residing in the same geographic region where the environmental samples were collected (Table 2). HAV was identified in 10 (61.1%) samples by real-time PCR method, five from serum and five from stools while three patients had positive results in both samples. From seven HAV confirmed cases (four women and seven men), mean age was 20.5 ± 11.0 years. Six individuals (54.5%) reported symptoms and three out seven individuals (42.8%) reported drinking unsafe water outside the country. HAV was detected at higher infection rates in patients aging more than 20 years (57.1%) compared to those aging less than 20 years (42.9%). Among HAV positive samples, high frequency was found in spring season [60% (6/10)] followed by summer [20% (2/10)], and winter [20% (2/10)].
HAV RNA-positive clinical samples.
AST, Aspartate aminotransferase; ALT, Alanine aminotransferase; N/D, Not done.
Demography, laboratory tests and season distribution.
During the same period, a total of 39 suspected hepatitis individuals from the same region provided 37 serum and 32 stool samples for HEV analysis (Table 3). Using real-time PCR, HEV was detected in 11 (15.9%) samples, being eight serum and three stool samples. From 11 HEV confirmed cases (six women and five men), mean age was 33.5 ± 10.3 years. HEV was detected at higher infection rates in patients aging above 25 years [70% (8/11)]. HEV was found predominant in autumn season (36.3%), followed by summer (27.3%), winter and spring (18.2% each). Among HEV infected individuals, we found four pregnant women and one immunosuppressed man. None of these patients presented severe liver disease due to HEV infection. The immunosuppressed patient had HEV RNA positivity by RT-PCR for two months after the first molecular diagnosis. However, after five months he presented undetectable HEV RNA, with complete clinical recovery. No individual presented HAV/HEV coinfection.
HEV RNA-positive clinical samples.
Demography and season distribution.
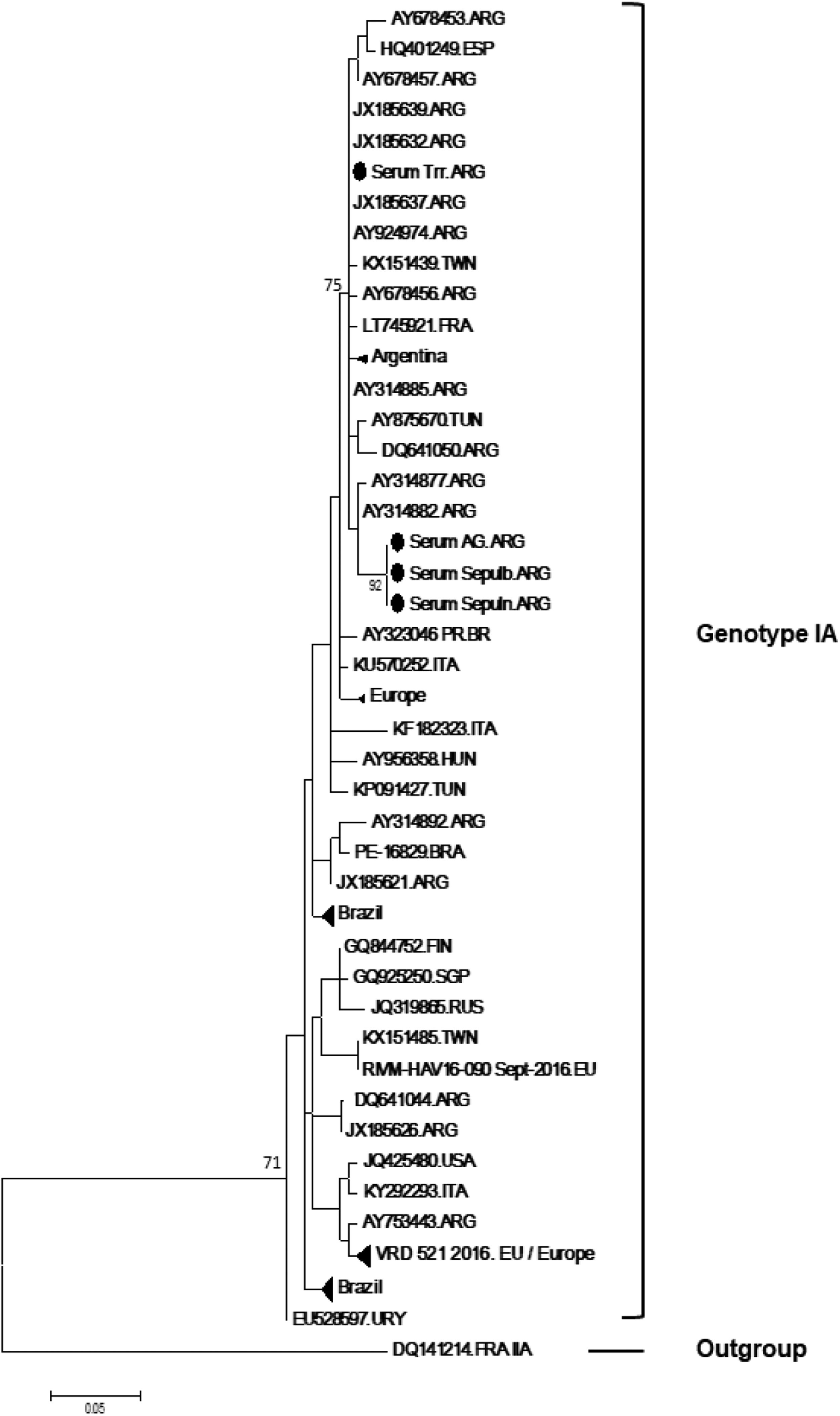
It was possible to sequence successfully 4/11 HAV clinical samples (Genbank accession numbers: MW400597-MW400600). From them, three were family members (father, 28 years-old, mother, 33 years-old and daughter, 1 year-old) (identified as 1, 2 and 6 in Table 1 and “Serum AG.ARG”, “Serum Sepulb.ARG”, Serum “Sepuln.ARG” in the phylogenetic tree) who were travelling through South America a few days prior the beginning of the symptoms. Phylogenetic and BLAST analyses demonstrated that all three samples were identical (100% genetic similarity) and clustered with other genotype IA samples from Bahia Blanca, Argentina[22] (genetic distance: 0.008) and Brazil (genetic distance 0.014).23 Viral isolates from Italy (Lazio, unpublished) are also in the same branch (Fig. 1). The 4th sequence did not show genetic relatedness with the family, displaying a genetic distance of 0.019, which is similar to the distance between this sample and other sequences from Argentina (Riachuelo River, Buenos Aires and Viedma, Río Negro).10,22
Phylogenetic reconstruction based on the VP1-2A gene sequences of HAV strains and reference strains by Maximum Likelihood method. Outgroup refers to a genotype IIA reference sequence. Bar refers to expected nucleotide substitutions per site. Black dots correspond to the study sequences (Genbank accession numbers: MW400597-MW400600). Only bootstrap values above 85% are shown (1,000 re-samplings) at each branch point.
Two HEV raw sewage samples were successfully sequenced and classified as genotype 3 (Genbank accession numbers: MW411790 and MW411791). Although a robust phylogenetic analysis has been limited by the restrict length of HEV sequences (∼150 bp), BLAST analysis suggested a closer similarity with sequences from France (genetic distance: 0.014).
DiscussionEnvironmental surveillance of water sources is an important tool for monitoring viral hepatitis transmission. Due to their high stability, to the expressive number of viral particles excreted by infected individuals and to the resistance of these viruses to wastewater treatment procedures, HAV and HEV may easily reach the water supply,12-14,24 leading to clinical outbreaks. Few studies have reported HAV and HEV detection in sewage and clinical samples over time. This is the first report about HAV and HEV circulation, at the same time, in environmental and clinical samples from Mendoza province, Argentina. In sewage samples, total HAV and HEV positivity were 39% and 22.5%, respectively. Despite the lack of data on the prevalence of HAV and HEV in environmental samples in Mendoza, our results showed higher HAV and HEV positivity when compared to other studies carried out in Argentina. Previous studies conducted in Argentina found 20.8% to 22.9% of HAV, respectively in sewage and recreational waters.12,13 HEV prevalence was 1.6 to 3.2% in river samples,14,8 6.3% in raw sewage[8] and 7.6% in recreational waters from Central Argentina13. External factors such as temperature, rainfall, different types of matrices studied, different study methodologies, extraction and/or viral concentration can mark the differences with our results. Moreover, HEV has been identified circulating in pigs in the province of Mendoza with a prevalence of 81% in pigs 2-3 months of age.25
HAV and HEV were detected in sewage in all seasons, demonstrating the circulation of these viruses over time. Higher HAV detection was found during the winter while HEV was observed more frequently in summer. The reasons for these differences are not fully understood. Carratala and Joost[24] observed that population density, annual potential evapotranspiration, and at a lesser extent the precipitation seasonality influenced the ecological suitability of viruses, such as HEV. Differences in viral prevalence according to precipitation were also observed by Bai et al.26 in the Philippines, where HAV detection was more frequent in the dry season. HAV seasonal distribution was also observed in central Tunisia, where higher prevalence was found in sewage samples during the winter.27
In this study, it is noteworthy that the higher detection of HAV in sewage in winter was followed by the emergence of clinical cases in the spring, when all four HAV sequenced cases were detected. Likewise, the higher incidence of HEV in the summer in sewage was accompanied by the detection of clinical cases in the autumn. Despite the unavailability of paired clinical and environmental sequences of each virus be a limitation of our study, our findings suggested that the circulation of these viruses in the environment may be the source of human infection in the next season.
In Mendoza, water from sewage effluents has been used for irrigation of food crops for the population's consumption ("A.C.R.E." zone - Special Area of Restricted Cultivation). However, sanitary requirements for the use of these effluents do not adopt virological parameters and do not include any treatment aiming the elimination of viral pathogens. As a result, workers from these areas of cultivation, as well as food consumers are exposed to potential contamination by enteric viruses. The detection of HAV and HEV in sewage effluents showed the circulation of both viruses in the province of Mendoza over time, despite Argentina's efforts to obtain good HAV vaccine coverage. All these factors demonstrate that HAV and HEV clinical outbreaks can occur once these viruses, which are present in the environment, find susceptible hosts. Alternatively, considering the transmission route and incubation time of these viruses, an increased environmental HAV and HEV detection in sewage could be consequence of an increase in clinical cases of hepatitis A and E possibly caused by the high number of tourists and immigrants in the province. In both cases, environmental monitoring of these viruses reveals the importance of including virological controls of post-treatment waters in the future, as these waters in a desert area are very often used for irrigation of agricultural products.
All four HAV sequences belonged to genotype IA which is common in clinical and environmental samples from South America.15,19,22,24 Of them, three were members of the same family and had recently returned from a South American trip where they reported consumption of unsafe water. Phylogenetic analysis showed 100% similarity among the sequences, demonstrating that they probably shared the same source of infection. The sequence representative of the three isolates presented close relatedness (genetic distance 0.008) with a sequence from Bahía Blanca (2002), Buenos Aires, Argentina.22 The sequence from the 4th case was dispersed elsewhere in the phylogenetic tree, clustering with genotype IA sequences from different regions of Argentina as Riachuelo River, Buenos Aires (2006) and Viedma, Río Negro (2004).10,22 The adoption of universal HAV childhood vaccination in 2005 in Argentina had led to epidemiological changes.10,11,28 However, it is important to note that all HAV infected individuals have not been vaccinated, including the one-year-old child, demonstrating the need to close the gaps in HAV immunization.
Regarding HEV, all environmental sequences belonged to genotype 3, the most prevalent genotype in several Latin American countries. This genotype was previously found in environmental, swine, and human samples from Argentina.7,13,14,29 In this study, HEV RNA was detected in 11 clinical cases, including four pregnant women and one immunosuppressed man. Despite none of these patients developed severe liver disease, the immunosuppressed patient presented HEV RNA positivity for a longer period, perhaps acting as a viral reservoir and a possible source of transmission. Similar findings were reported by Ankcorn et al.30 in a study conducted in England and Wales, where persistent viremia was observed in immunocompromised patients. It is known that pregnant women present high rates of progression to fulminant hepatic failure due to HEV infection.3,6,31 Recently, Tissera et al. have found HEV seropositivity rate of 8.4% among pregnant woman from Córdoba, Argentina. In agreement with our results, no severe cases were observed by the authors.31
The presence of HAV and HEV in sewage and clinical samples reinforces the idea of the viral circulation in Mendoza, a touristic province from Argentina, despite the preventive measures as improvements in sanitation, water supply and the HAV National Vaccination Program.
Regarding the sensitivity of the techniques employed, it is known that viruses dispersed in water sources are highly diluted. Moreover, many substances can strongly inhibit PCR in environmental samples.32 In this study, two different PCR methods have been employed for HAV and HEV RNA detection in clinical and environmental samples, resulting in variable positivity according to the method. It can be explained by several factors such as the size and nucleotide composition of the amplified genomic region, sensitivity of the technique, presence of inhibitors, etc. These discrepancies reinforce the importance of using more than one molecular test for the detection of RNA viruses in environmental samples.
This study has some limitations: since sewage samples were collected over a period of one year, it was not possible to assess seasonality in viral detection in this study. Regarding clinical cases, it was not possible to know if the family members acquired hepatitis A in Argentina or in any of the countries to which they had travelled. Information about which countries have they travelled to was also not available. Furthermore, the number of clinical samples is very reduced, limiting reliable conclusions. Nevertheless, this study provides important data on the circulation of HAV and HEV in Mendoza, Argentina, may contributing to future decisions, such as the adoption of virological parameters for the use of effluents.
ConclusionIn conclusion, high prevalence of HAV and HEV were observed in raw sewage and in clinical samples collected between 2016 and 2017. HAV and HEV were detected in all seasons in both environment and clinical cases. These findings thus reinforce the importance of continuous environmental surveillance to reduce burden of transmission.
Data availabilityAll relevant data are available in the manuscript. Details are available from the corresponding author upon request.
Authors’ contributionsConceptualization: LMV, CE, VSP, ILC. Supervision: LMV, VSP, CE. Methodology: ILC, NRA, JEG, LMV, VSP. Data analysis and interpretation: ILC, VSP, CE, LMV, BVL. Writing, Review and editing: LMV, BVL, CE. All authors have read and agreed to the published version of the manuscript.
This research was supported by the Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ), Brazilian National Counsel of Technological and Scientific Development (CNPq), CAPES, MINCYT, CONICET.