Sepsis is one of the most common reasons for hospitalization. This condition is characterized by systemic inflammatory response to infection. International definition of sepsis mainly points out a multi-organ dysfunction caused by a deregulated host response to infection. An uncontrolled inflammatory response, often referred to as “cytokine storm”, leads to an increase in oxidative stress as a result of the inhibition of cellular antioxidant systems. Oxidative stress, as well as pro-inflammatory cytokines, initiate vascular endothelial dysfunction and, in consequence, impair microcirculation. Microcirculation damage leads to adaptive modifications of cell metabolism. Moreover, mitochondrial dysfunction takes place which results in increased apoptosis and organ damage. Non-coding RNA fragments, especially miRNA molecules, may play an important role in the pathomechanism of sepsis. Numerous studies have indicated altered expression of various miRNAs in sepsis. miRNAs can be used as markers in the diagnosis and prognosis of disease development. In turn, intracellular miRNAs regulate the TLR4/NFκB pathway responsible for the expression of pro-inflammatory cytokine genes involved in the inflammatory response in sepsis. The understanding of detailed molecular mechanisms leading to organ damage can contribute to the development of specific therapy methods thereby improving the prognosis of patients with sepsis.
Sepsis, a syndrome of physiologic, pathologic, and biochemical abnormalities induced by infection, is one of the key public health concern consuming huge funds around the world and it is the main cause of deaths in intensive care units [1]. Significant advances in critical care medicine have defined sepsis as an organ dysfunction resulting from the dysregulation of the immune response to infection. The incidence of this type of disorder is still constantly rising. Morbidity significantly correlates with the aging of societies and presence of other comorbidities, especially of the metabolic type [2]. The prognosis is worse when the aforementioned correlations are strong and the patient is diagnosed too late. Although sepsis can occur in any age group, its risk is definitely greater in patients over 60 years of age. Moreover, sepsis develops rapidly as a result of a poorly controlled, multidirectional response of the body to an external factor, i.e. pathogen, and it can be further aggravated by endogenous factors or concomitant diseases [1–3].
Over the last 30 years, the concept of sepsis has evolved several times. The modification of the approach to sepsis definition was crucial for the correct diagnosis, implementation of appropriate therapy and conducting of controlled clinical trials on sepsis. The definition of sepsis [4] developed in 1991 by medical societies of critical care medicine during an US conference introduced the new medical term SEPSIS-1, or Systemic Inflammatory Response Syndrome (SIRS), and clinical criteria for its diagnosis based on basic life parameters. The dissemination of SEPSIS-1 definition has aroused much controversy, especially in the context of the SIRS criteria which were chosen arbitrarily. However, from a clinical point of view these criteria proved to be too sensitive and non-specific. Comments concerning the lack of specificity of the SIRS definition had not been taken into consideration until a consensus conference held in 2001 [5], which gathered experts not only from the US but also from Europe. The new definition of SEPSIS-2 has been expanded to include a list of symptoms and test results of patients with sepsis. Succeeding years brought new modifications to the definition of sepsis due to the awareness that the use of SIRS symptoms in the initial diagnosis of sepsis was too sensitive and not specific. In 2016, the Council of Experts on Sepsis (SEPSIS-3) decided to exclude SIRS from the definition of sepsis, and to focus on life-threatening organ dysfunction resulting from body's abnormal or dysregulated response to infection finally leading to tissue and organ damage [6]. As a results of experts’ agreement concerning SEPSIS-3, a detailed definition of sepsis was established, but also other definitions of severe sepsis, multiple organ dysfunction syndrome (MODS), and septic shock, i.e. severe sepsis which does not respond to the use of fluid therapy and hypoperfusion have been introduced.
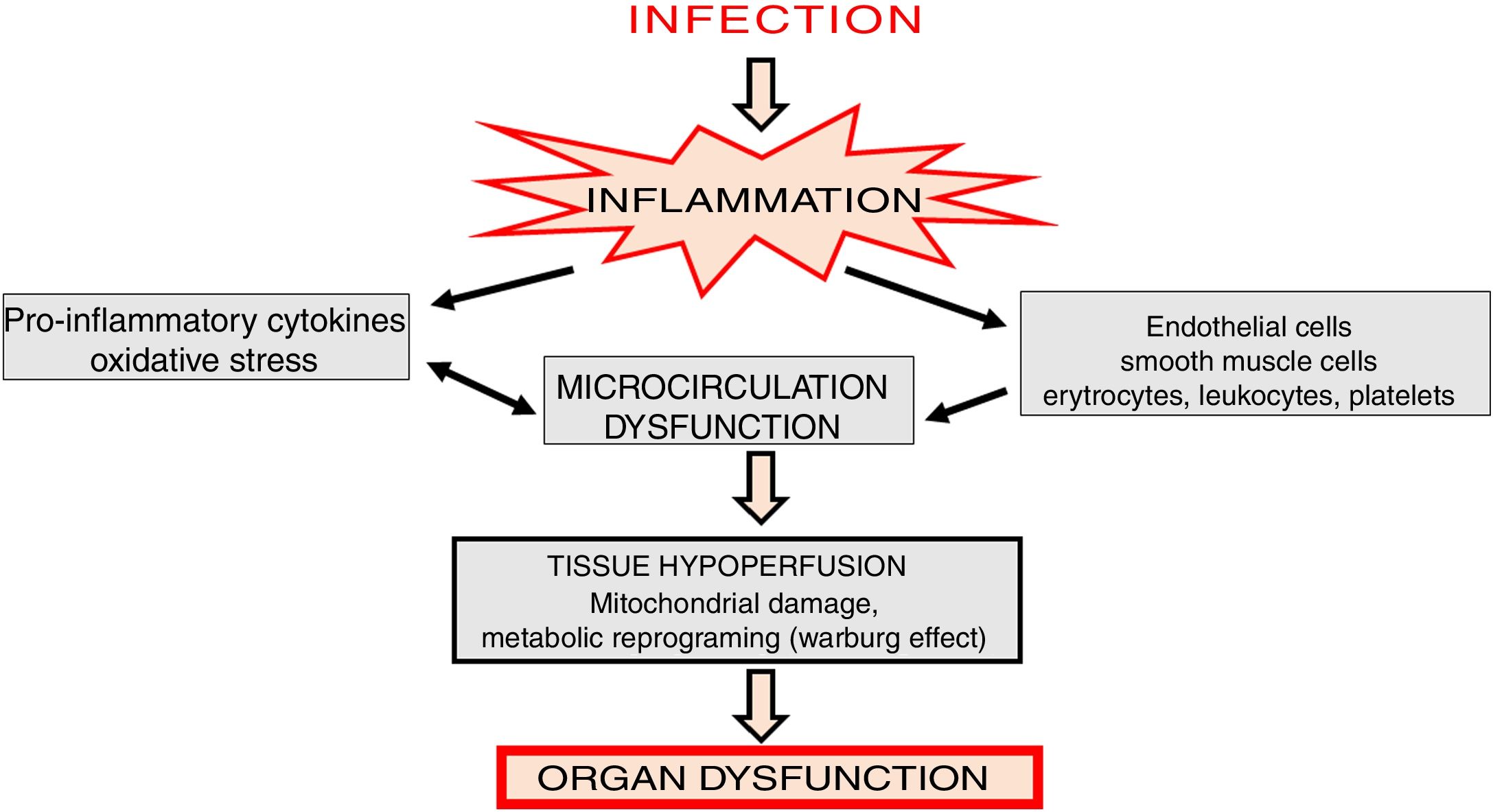
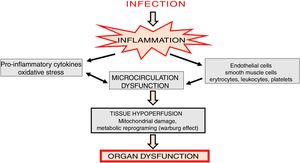
Microcirculation in sepsisSepsis is associated with deep changes in microcirculation. The mechanisms leading to these changes are comprehensive and involve primarily vascular endothelial dysfunction, glycocalyx degradation [7], rheological changes in red blood cells related to their decreased ability to change shape [8] and lack of balance between vasodilators and vasoconstrictors [9]. Deficient oxygen supply to tissues is the basic hemodynamic effect observed in sepsis.
In the initial stages of sepsis, pro-inflammatory cytokines are released and an intense inflammatory response is generated causing microcirculation changes [10]. In such state, disorders of almost all cellular elements of microcirculation, including endothelial cells, vascular smooth muscle cells, erythrocytes, leukocytes, platelets and parenchymal cells are observed [11] (Fig. 1). Activation of leukocytes induces their adhesion, rolling and transmigration into tissues, which additionally intensifies inflammatory state, activates platelets as well as the coagulation cascade and complement system [12].
Nitric oxide (NO) is an important element of the microcirculation dysfunction mechanism. In sepsis, NO synthesis is generally increased. However, considerable amounts of NO are produced by the inducible form of NO synthase (iNOS) which generates reactive nitrogen species (RNS) thus enhancing oxidative stress [13]. The significance of this mechanism is reflected by the improvement of microcirculation and restoration of renal function after administration of a selective iNOS inhibitor [14]. The induction of iNOS is accompanied by inhibition of endothelial synthase (eNOS) which results in a significant reduction in the bioavailability of NO. Therefore, in sepsis, the protective role of NO associated with the inhibition of platelet aggregation and local relaxation of vascular smooth muscles is decreased, which in consequence leads to microcirculation dysfunction [15].
Altered blood flow in the microcirculation leads to a significant reduction in tissue perfusion, which depends on two basic mechanisms: diffusion and convection. The diffusion process is determined by vessels density, while convection is related to the flow rate and hemoglobin saturation [16]. Sepsis is associated with a significant increase in the number of obstructed capillaries [17] which results in organ hypoxia. In consequence, there is metabolic reprogramming, involving the shift in ATP generation from oxidative phosphorylation to oxygen glycolysis (Warburg effect), inhibition of mitochondrial respiratory chain, and cell cycle arrest (Fig. 1).
Cytokine storm in sepsisCytokines are a large group of low molecular weight proteins (mostly <40 kDa) which ensure communication between cells. Although synthesized by different cells, cytokines are predominantly produced by macrophages and helper T lymphocytes (Th). Cytokines act on the cells that release them, as well as on adjacent and very distant cells, in auto-, para- and endocrine manner, respectively. They are often synthesized in a cascade, as one cytokine stimulates target cells to produce other cytokines. This sequential release of cytokines is called the cytokine cascade [18]. Cytokines also play a pleiotropic role in the regulation of both innate and adaptive immune systems [19].
Increased release of prototype inflammatory cytokines, such as tumor necrosis factor (TNF-α), interleukins (IL-1β, IL-6) and monocytic chemotactic protein (MCP-1) leads to many immunopathological processes in sepsis, which is often referred to as ‘cytokine storm’ [3,20], although this term is not precisely defined. A cytokine storm is a potentially dangerous immune response which forms a positive feedback loop between cytokines and immune cells. When the immune system is fighting the pathogen, cytokines transmit a signal to lymphocytes and macrophages to move towards the site of inflammation. Moreover, cytokines stimulate these cells to produce effector cytokines. In sepsis, this coupling gets out of control, which results in the excessive activation of immune cells in one space and consequent damage of the inflamed organ. Alternatively, cytokines released into the bloodstream can also induce damage to distant organs via activation of immune cells [21].
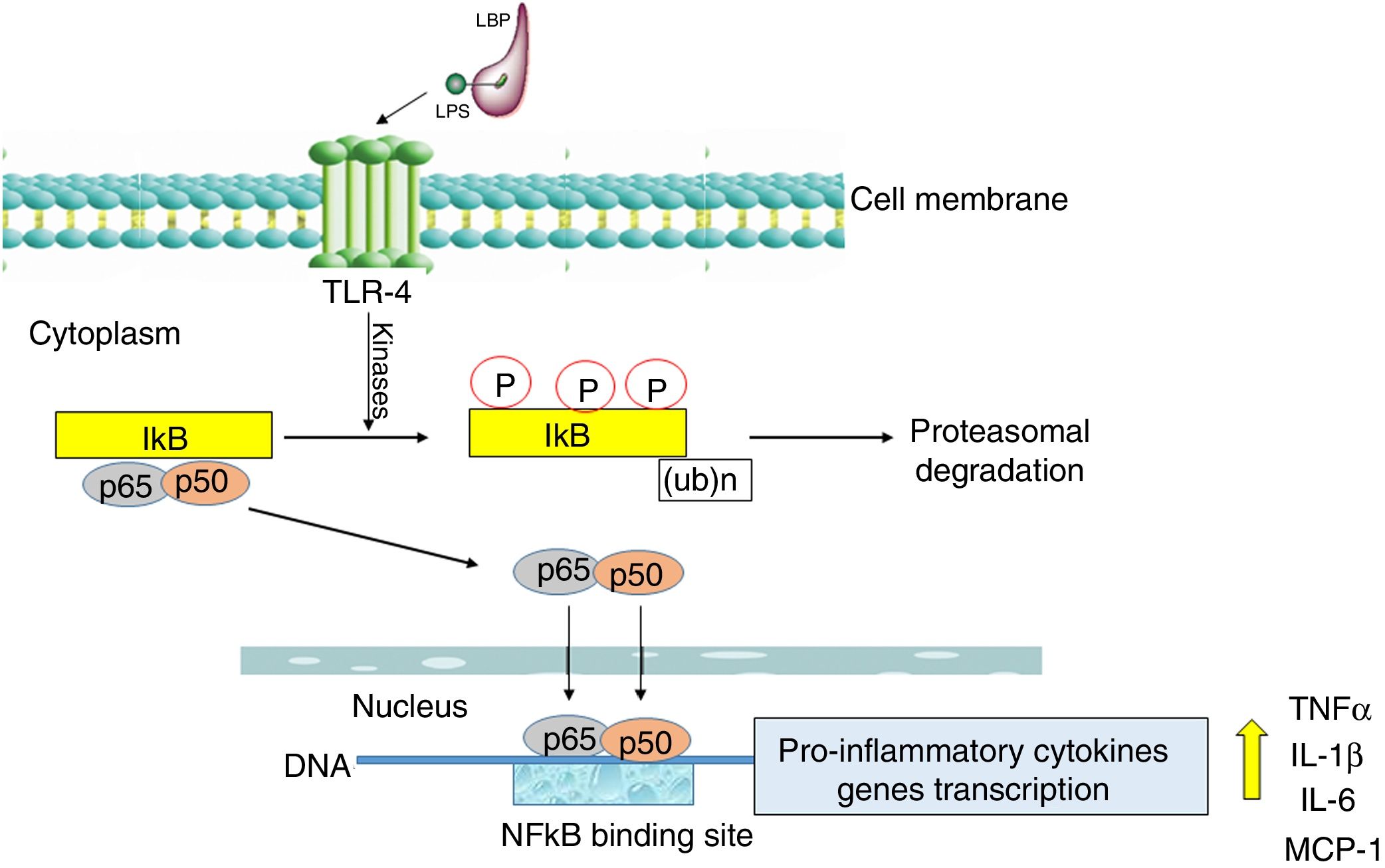
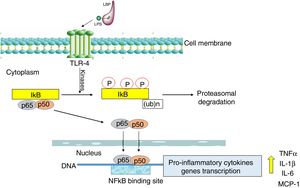
Nuclear transcription factor (NFκB) plays a fundamental role in the onset of a cytokine storm due to its ability to induce the expression of pro-inflammatory genes. NFκB factor is localized in cell cytoplasm where it is attached to its inhibitory protein, IκB. NFκB is activated by many stimulators, the most important being bacterial pathogens recognized by Toll-like receptor 4 (TLR-4) as well as pro-inflammatory cytokines recognized by specific membrane receptors (e.g. TNFR, TNF receptor) [22].
The activation of the TLR-4 receptor by a complex of lipopolysaccharides (LPS) with a binding protein (LPS/LBP) leads to the stimulation of one or more redox state-dependent kinases which specifically phosphorylate the inhibitor (IκB). Polyubiquitination is followed by the degradation of the inhibitor in proteasomes (Fig. 2). The released p65:p50 dimer translocates to the cell nucleus, where it regulates the synthesis of many pro-inflammatory molecules [23]. NFκB also activates the expression of cyclooxygenase-2 (COX-2, EC 1.14.99.1) as well as inducible iNOS [24]. The significant role of NFκB in the inflammatory and immune response makes it an important possible target for pharmacological therapeutic interventions designed to prevent multi-organ damage in the course of sepsis [25]. The effect of intervention targeted at the inhibition of NFκB activation was studied in rodents in a LPS-induced sepsis model. Moreover, it was demonstrated that curcumin, a component of curry, inhibited NFκB activation pathway and alleviated liver damage in mice with LPS-induced sepsis [26].
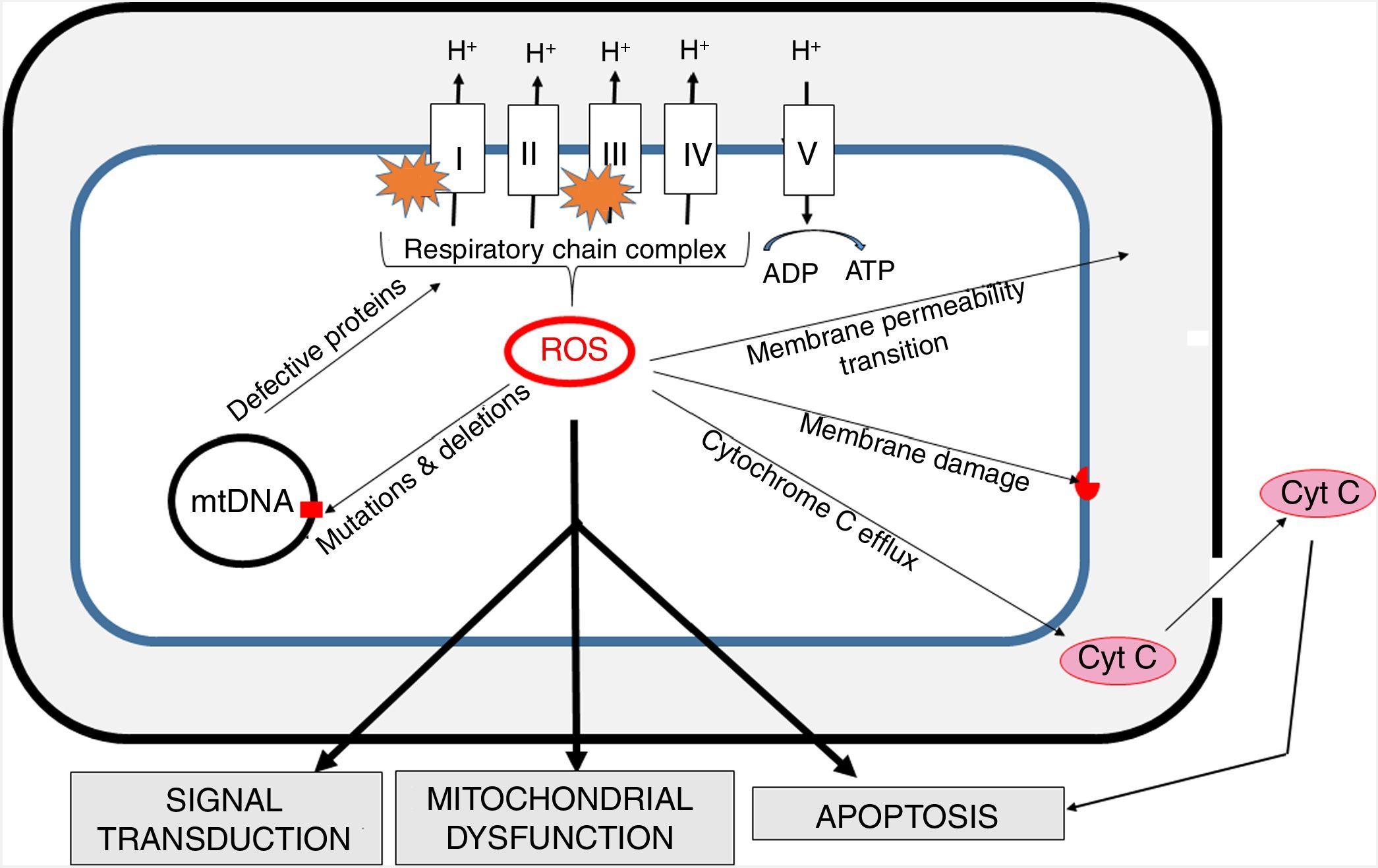
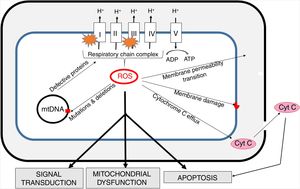
Oxidative stress and mitochondria in sepsisThe inner mitochondrial membrane is a large, impermeable structure containing enzymatic respiratory chain complexes (I-IV) and the ATP synthase system (V complex). ATP synthesis is coupled with electron transport (via complexes of the respiratory chain), which is accompanied by proton (H+) translocation to the intermembrane space and the generation of difference in potentials on both sides of the membrane. In four one-electron steps, the oxygen molecule is reduced to water. This process involves also the generation of reactive oxygen species (ROS) as a result of “electron escape” and one-electron reduction of oxygen to superoxide anion (O2) (Fig. 3).
The generation of intra-mitochondrial ROS and its consequences for the functioning of mitochondria.
ROS formed in mitochondria promote oxidative damage to mitochondrial proteins, membranes and mtDNA. These damages result in the outflow of cytochrome C into the cytosol and consequent increase in apoptosis. Increased permeability of inner mitochondrial membrane enables the transport of many different small molecules. Mitochondrial ROS affect signal transmission processes, which may alter some cell functions. mtDNA - mitochondrial DNA.
The mitochondrial respiratory chain is the main source of cellular ROS and at the same time mitochondria are the primary target of their activity [27]. ROS play an important role in cell signaling and under normal conditions their activity is strictly controlled by the interaction with antioxidant systems. The formation of ROS by mitochondria is crucial in the functioning of the cell, as they are involved not only in energy generation, but also in calcium and iron homeostasis [28].
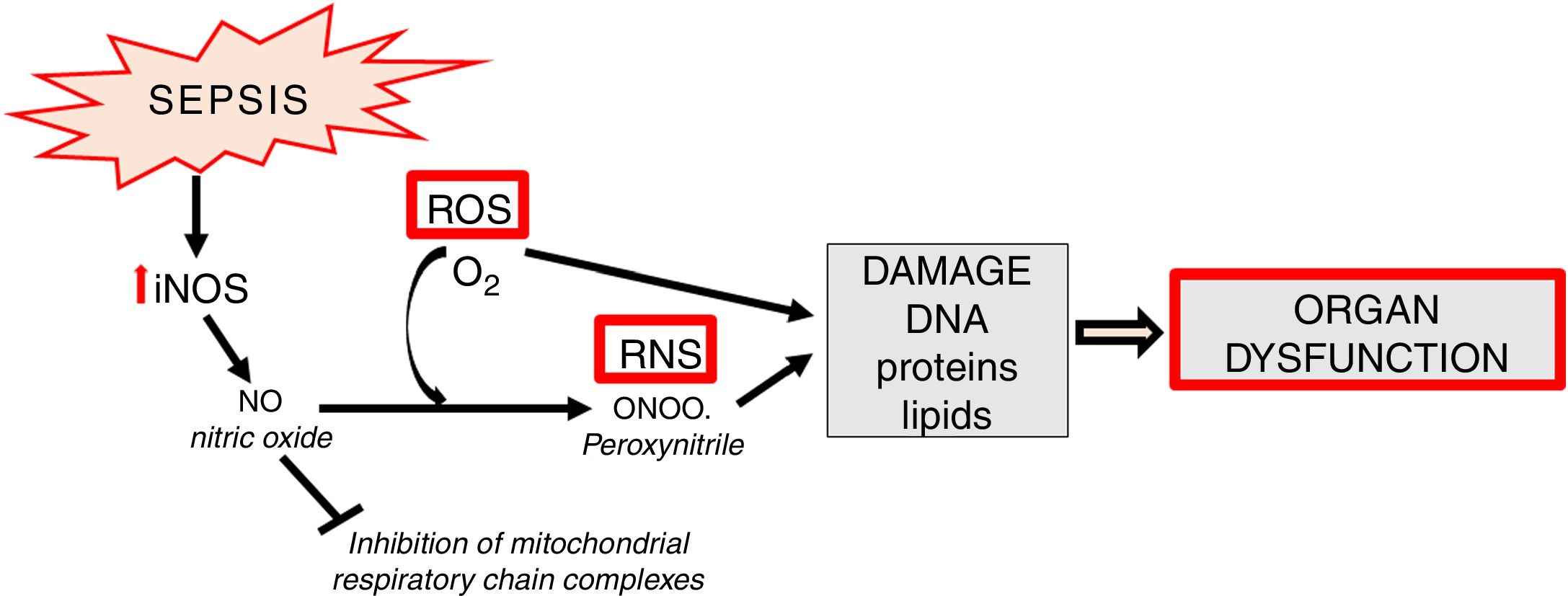
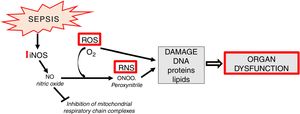
Apart from the generation of ROS in mitochondria, increased synthesis of NO which contains an unpaired electron (in fact it is a free radical) is an important element of oxidative stress. NO also undergoes conversion into other, extremely active nitrogen compounds, primarily in reaction with the superoxide anion, forming peroxynitrite (ONOO) (Fig. 4).
In sepsis, de novo enhanced synthesis of NO by iNOS is observed. Despite the fact that superoxide anion is quickly converted into H2O2 by superoxide dismutase (SOD, E.C.1.15.1.1) and then by catalase into the molecule of water - the reaction of peroxynitrite generation is significantly enhanced. The resulting ONOO− is mainly responsible for the cytotoxic effects of reactive nitrogen species (RNS) [13]. RNS induce a number of dangerous effects through oxidation, nitration, nitrosylation of proteins, nucleic acids, lipids and endogenous antioxidants, e.g. glutathione [29] (Fig. 4). Some studies suggest the existence of mitochondrial NO synthase, however, this thesis is controversial [30,31].
In sepsis, NO is produced by various cells, including activated macrophages, neutrophils and lymphocytes [32]. Many molecules involved in septic inflammation, such as TNF-α, interferon-γ (IFN-γ) and IL-1β participate also in the induction of iNOS through the activation of IκB degradation and transcription of the iNOS gene [33].
Cells have developed defense mechanisms against mitochondrial dysfunction such as the system of endogenous antioxidants, mitochondrial dynamics as well as processes of removal of damaged organelles and biogenesis of new ones. In order to counteract oxidative damage, mitochondria contain high concentrations of antioxidants, i.e. substances present in low concentrations reduce and/or protect substrates against oxidative modifications [34]. Antioxidant functions involve ROS uptake. There are two types of antioxidants: enzymatic and non-enzymatic [35]. Superoxide dismutase (SOD), catalase (CAT, EC 1.11.1.6) and glutathione peroxidase (GPx, EC 1.11.1.9) are important antioxidative enzymes [36]. SOD catalyzes the conversion of the O2 radical into H2O2 and oxygen [34]. The level of SOD in plasma is significantly decreased in patients with severe sepsis [36]. Two forms of SOD are present in mammalian cells: CuZnSOD (SOD1) and MnSOD (SOD2). SOD 1 requires the presence of copper and zinc as cofactors and it is present mainly in the cytosol, while SOD2 is manganese-dependent and is localized primarily in mitochondria. H2O2 formed in the reaction catalyzed by SOD is then detoxified by CAT or GPx. CAT catalyzed reaction produces water and oxygen. GPx converts H2O2 to water in a reaction in which glutathione (GSH) is oxidized to disulphide (GSSG) and then reduced to GSH by glutathione reductase (GR, EC 1.8.1.7). Both GPx and GR require the presence of selenium [37]. Apart from selenium, other microelements, such as: manganese, copper and zinc are important elements of antioxidant systems. Moreover, vitamins (A, C, E), enzymatic cofactors and many endogenous substances are important non-enzymatic antioxidants [35]. The balance between antioxidative (discussed above) and oxidative systems is significantly disturbed in sepsis, with a shift towards oxidation, which leads to the generation of oxidative stress.
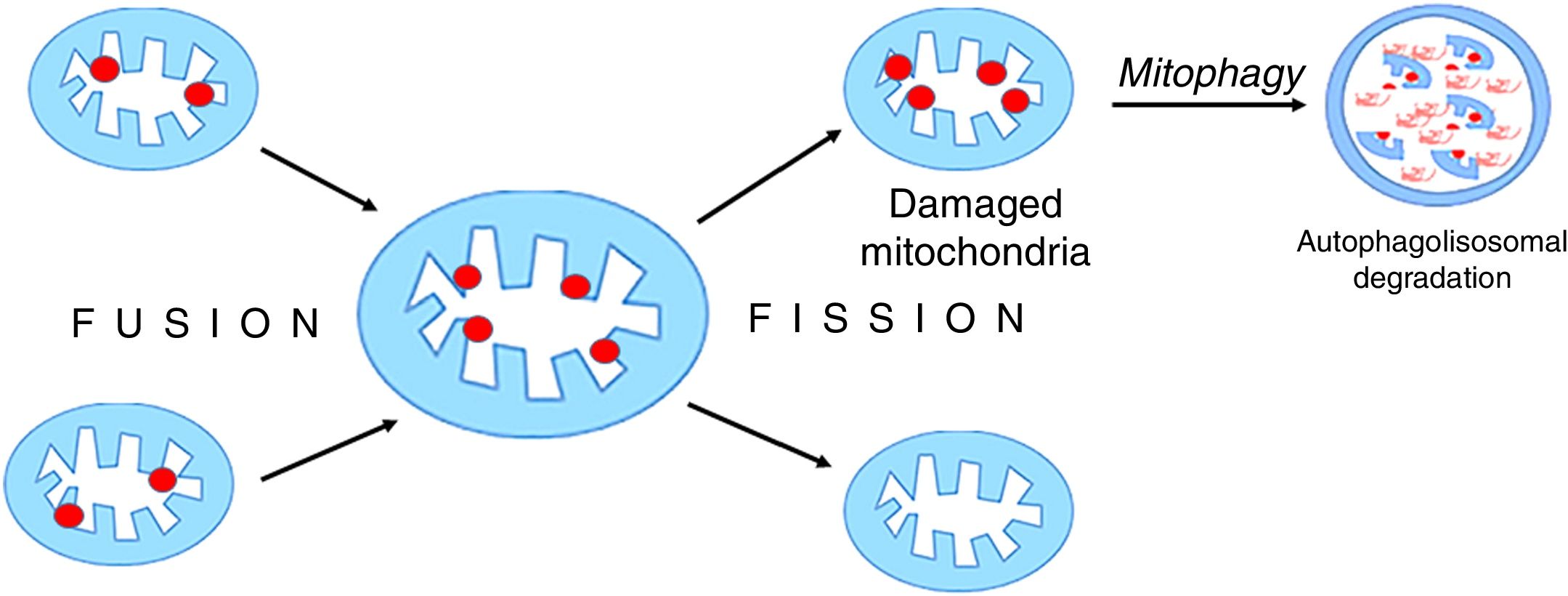
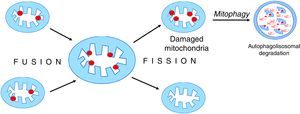
Mitochondria are dynamic organelles that undergo continuous regular fusion and disintegration cycles [38]. The fusion of damaged mitochondria followed by their asymmetrical disintegration leads to the recovery of their full functionality [27,39,40] (Fig. 5).
Mitochondrial fusion and disintegration.
Damaged elements present in different organelles are gathered as a result of fusion into one mitochondrion. Asymmetric decay leads to the formation of functional organelles and mitochondria in which all damages are accumulated. These dysfunctional mitochondria are removed by autophagy.
Mitochondrial fusion and decay must be strictly balanced, otherwise, these uncontrolled processes can pose a great threat to cell functioning and lead to its death [38]. There is still little data on mitochondrial fusion and decay in critical states. Increased levels of markers reflecting mitochondrial dynamics were found in post mortem liver biopsies, but not in vivo in critically ill patients [41]. Studies on rabbits used as a model of critical illness have revealed severe mitochondrial dysfunction in liver and kidney of these animals, and indicated a significant increase in mitochondrial intimal membrane fusion protein (OPA1, optic atrophy 1) only in the liver. At the same time, decrease in mitofusin 2 (MTF-2), a protein which regulates metabolic processes and is involved in the fusion of outer mitochondrial membrane, has been observed [42]. However, the level of breakdown markers remained unchanged in both aforementioned organs. The results of these studies may suggest that mitochondrial ability to fuse and decay exhibits organ diversity.
Removal of dysfunctional mitochondria requires the replenishment of their population by biogenesis. Mitochondrial biogenesis depends on the activation of the nuclear and mitochondrial transcription system [43]. PGC-1α (peroxisome proliferator-activated receptor gamma-coactivator 1 alpha) which activates nuclear respiratory factors 1 and 2 (NRF1, NRF2) has been identified as a key element of the biogenesis process [44]. NFR1 and NFR2 induce important transcription factors, including mitochondrial transcription factor A (TFAM) and nuclear-encoded mitochondrial proteins - subunits of respiratory chain complexes [27]. This comprehensive transcription program leads to mtDNA replication and the synthesis of new proteins necessary for the formation of new mitochondria. This program is extremely metabolically expensive, as it requires great expenditure of energy [43].
In transgenic mice models of sepsis, increased levels of the hepatic mitochondrial biogenesis markers PGC-1α, NRF1 and NRF2 were observed, which was associated with recovery of metabolic activity and improvement of clinical status [45]. In contrast, in critically ill rabbits, no difference in markers of liver and kidney mitochondrial biogenesis was observed between animals which survived and those which did not survive [42]. In patients with sepsis who survived, no change in mitochondrial protein synthesis was observed in vivo, despite the increase in mRNA levels of mitochondrial transcription factors [46]. The aforementioned data seem to indicate that in critical states, the process of mitochondrial biogenesis occurs only to a small extent, due to i.a. energy shortage (impaired ATP synthesis).
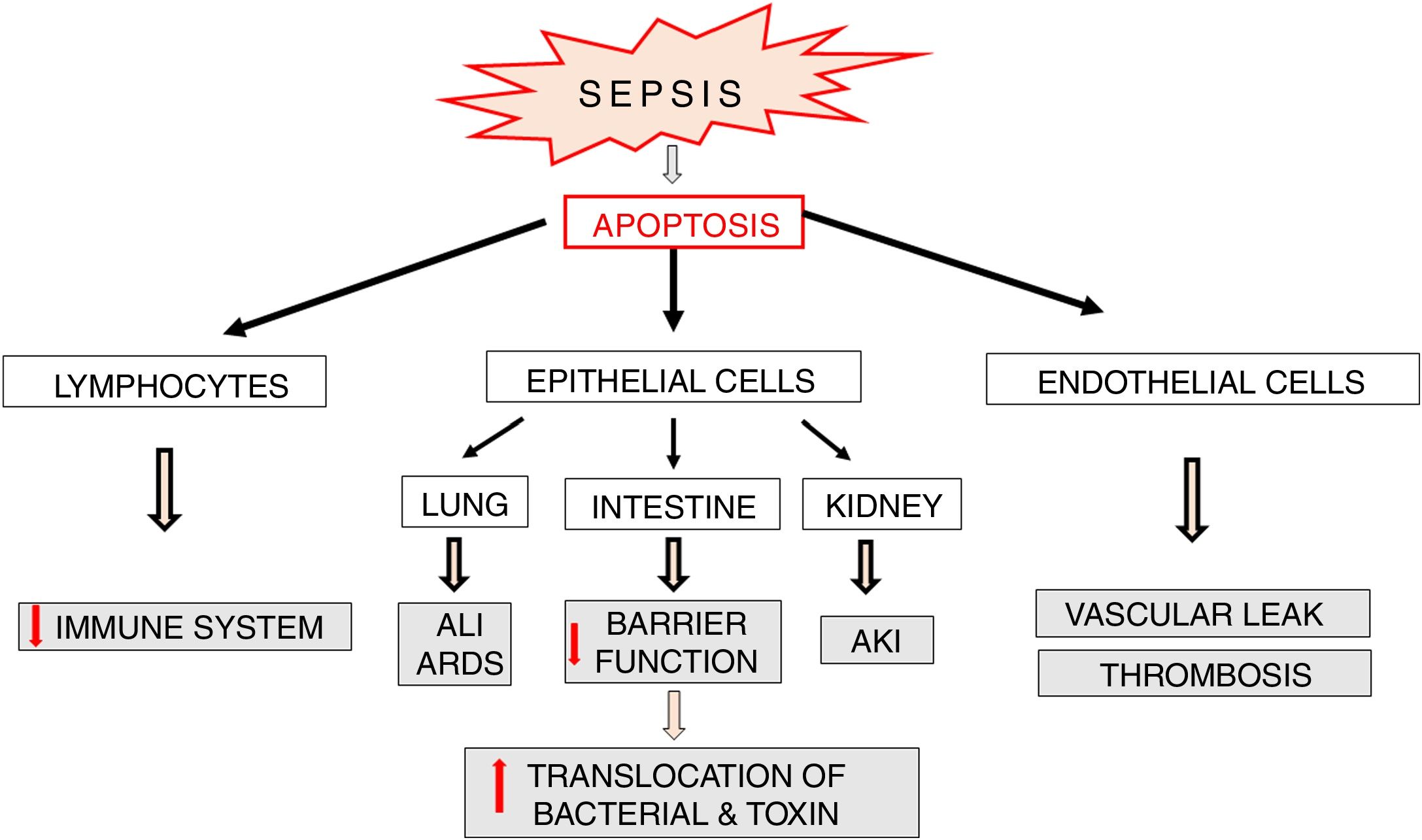
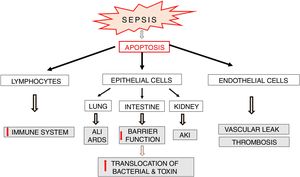
Apoptosis in sepsisApoptosis is a process which plays an important role in the pathophysiology of sepsis (Fig. 6). Lymphocyte apoptosis plays a role of a potential factor involved in immunosuppression and mortality in sepsis. Increased apoptosis of T and B lymphocytes was observed in patients who died of sepsis [47]. The above mentioned results of clinical trials confirm earlier observations of these authors, which indicated a significant increase in lymphocyte apoptosis in a model of sepsis induced by cecal ligation and puncture (CLP) in mice [48]. Despite the fact that the death of adaptive immune system cells can be beneficial since it limits inflammatory reaction associated with sepsis, intense apoptosis of immune cells leads to reduced ability to fight the invasive pathogen. It seems that lymphocyte apoptosis can result in weakening of the immune response and predispose to septic death. This suggestion is confirmed by the results of studies on transgenic mice with increased expression of anti-apoptotic Bcl-2 protein. In the model of CLP-induced sepsis, protective actions were observed in these animals in relation to T cells and these effects were associated with significantly improved survival [49]. This suggests that apoptosis of immune cells plays an important role in the development of sepsis possibly affecting its adverse course.
Parenchymal cells, including intestinal and lung epithelial cells, also undergo intensive apoptosis in experimental sepsis models [50]. The loss of intestinal epithelial cells may lead to a significant impairment of their function as a barrier and facilitate bacterial translocation into the blood and/or lymphatic system resulting in increased antigen presentation and massive immune response, which directly affect survival [51,52] (Fig. 6). Apoptosis of pulmonary epithelial cells leads to the development of acute lung injury (ALI). This pathology is directly caused by inflammation, aspiration or trauma but also by hemorrhagic shock and multi-bacterial sepsis [53,54]. The silencing of Fas receptors on membranes of lung epithelial cells of mice with hemorrhagic shock leads to limitation of ALI via blockade of apoptosis and thereby inhibition of histological rearrangement of the lung [50].
The role of miRNA in sepsisMicroRNA molecules (miRNAs) are a group of small, non-coding RNAs with the length of 21–26 nucleotides. miRNAs are derived from primary transcripts (pri-miRNAs) arising from non-coding regions of protein encoding genes or from intergenic regions. Primary transcripts are then cleaved by a Drosh nuclease/DGCRB complex and pre-miRNA molecules are formed. Pre-miRNA are transported to the cytoplasm by the nuclear transporter – exportin 5 [55]. Mature miRNA duplexes are formed in the cytoplasm with the help of Dicer nucleases. miRNA functioning requires the incorporation of mature molecules into the RISC (RNA-induced silencing complex). This complex detects complementary sequences in the mRNA at the 5′ end of the untranslated region. The attachment of miRNA results in destabilization and degradation of mRNA or inhibition of its translation [55]. Therefore, miRNA molecules act as post-transcriptional repressors regulating gene expression.
miRNA molecules can be released from cells into plasma where they remain stable. They are resistant to the degradation by exo- and endogenous RNases as well as to changes in pH and temperature. These properties are related to the fact that access of these factors to miRNAs is limited due to their attachment to membranes of microbubbles and exosomes [55]. Microbubbles are formed by direct detachment from cell membrane and their size is approximately 100−1000 nm. Exosomes are much smaller (30−100 nm) and are formed from multivesicular bodies; following their binding to the cell membrane they undergo exocytosis. Exosomes have been detected in various body fluids: plasma, serum, urine, breast milk and saliva [56]. miRNAs can also be released following tissue damage or apoptosis in the form of apoptotic bodies [57]. Also Argonaute 2 (AGO2) protein and high density lipoproteins (HDL) are carriers of miRNA [58].
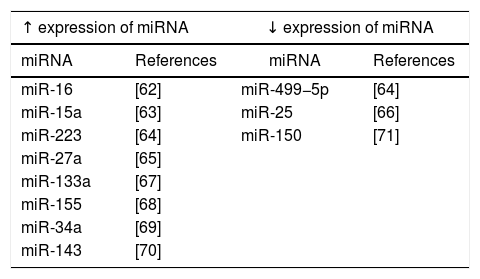
Increasing number of observations indicate that miRNAs are involved in the regulation of many different metabolic pathways, including innate immune processes, apoptosis and mitochondrial functioning, which are important in the pathophysiology of sepsis [59]. Analyses of miRNA expression profile in patients with sepsis have been performed with the use of microarrays [60] (Table 1). These studies have shown that miRNA expression occurs in the course of various pathological infection processes [61]. It has also been shown that the deregulation of miRNA expression correlates with symptoms of severe inflammation as well as sepsis [62–66].
In the course of sepsis, also other miRNA molecules, including miRNA-25, miRNA-133a, miRNA-146, miRNA-150 and miRNA-223 are deregulated and thus they may be used as markers in different stages of sepsis [59,72]. Vasilescu et al. demonstrated a significant decrease in miRNA-150 expression in the early stages of sepsis [71]. Moreover, miRNA-15a, miRNA-16, miRNA-122, miRNA-146a, miRNA-223, miRNA-499-5p can be used as diagnostic biomarkers, while miRNA-93b, miRNA-4835p and miRNA-574p can act as prognostic markers [63,64,72]. The vast involvement of miRNA molecules in excessive immune response, immunosuppression or apoptosis confirms their role in various stages of sepsis development and suggests their utility in the monitoring of disease course [73].
Recent studies have shown that intracellular miRNAs play a significant role in the regulation of TLR4/NFκB pathway which is responsible for the immune response. Chen et al. [74] found that let-7i regulated TLR4 expression via post-transcriptional suppression in cultured human cholangiocytes [74]. They observed that infection of culture with Cryptosporidium parvum resulted in the decrease in let-7i expression. Decrease in let-7i expression led to increase in TLR4, while its overexpression reduced the level of TLR4 protein. These data seem to suggest that miRNAs can post-transcriptionally regulate response to general bacterial infection.
Studies carried out by Wendlandt et al. [75] clearly indicate that molecules belonging to miRNA-200 family (miRNA-200a, miRNA-200b and miRNA-200c) are important regulators of the TLR4/NFκB signaling pathway. They demonstrated the repression of NFκB activity after the transfection with miRNA-200b and miRNA-200c mimetics, while such effect was not observed in case of miRNA-200a mimetics. Xu et al. [76] obtained similar effects for miRNA-149 family.
Furthermore, apart from the negative regulation of the TLR4 signaling pathway (Fig. 2), miRNAs can also directly affect the synthesis of pro-inflammatory cytokines. El Gazar and McCall [77] demonstrated the repression of TNF-α synthesis by miRNA-221, miRNA-579 and miRNA-125b. The transfection of THP-1 cells (human leukemia monocytic cell line) with miRNA-221 promoted the degradation of TNF-α mRNA, while their transfection with miRNA-579 and miRNA-125b resulted in a significant reduction in TNF-α protein levels. The results of these studies are consistent with previous observations of Tili et al. [78]. It seems that miRNAs are important elements of mechanisms activating inflammatory state in sepsis.
Summary and perspectivesSepsis is a complex clinical syndrome characterized by significant heterogeneity, in which organ damage is the main cause of high morbidity and mortality. Impaired vascular endothelial function resulting in microcirculation disorders and tissue hypoxia, is the primary mechanism stimulating the process of organ damage. These changes are triggered by an excessive reaction to infections reported as a cytokine storm. Inflammatory cytokines are the main element of the group of molecules associated with damage. Tissue hypoxia, as well as increased oxidative stress (ROS and RNS generation), lead to mitochondrial dysfunction and adaptive reprogramming of cell metabolism (Warburg effect). At that time, a significant increase in apoptosis leading to the loss of organ functioning is observed. The generally accepted thesis that the inflammatory response in sepsis occurs via the TLR4/NFκB pathway seems to be no longer justified as administration of anti-inflammatory drugs, such as corticosteroids or TNFα antagonists and IL-1 receptors do not bring the expected results in patients with sepsis [79].
Non-coding RNA molecules, primarily miRNAs, seem to play an important role in the development of sepsis. Many miRNA molecules are involved in the regulation of the inflammatory response by affecting the TLR4/NFκB pathway. Intracellular miRNAs can therefore be a potentially effective target for the treatment of sepsis. Recently, it has been demonstrated that inhibition of miRNA-23b expression prevents the development of myocardial dysfunction in late sepsis [80]. At the same time, miRNA molecules released from cells seems to be promising molecular biomarkers enabling sepsis monitoring. However, further studies of mechanisms responsible for organ damage in sepsis are necessary. Knowledge of these detailed mechanisms will enable full understanding of complex pathogenesis of sepsis and the development of procedures limiting the progression of organ damage.
Conflict of interestThe authors declare no conflicts of interest.