During SARS-CoV-2 (severe acute respiratory syndrome coronavirus-2) pandemic, the etiologic agent of COVID-19, several studies described the involvement of other tissues besides the respiratory tract, such as the gastrointestinal tract. Angiotensin-converting enzyme-2, the functional virus host cell receptor expressed by organs and tissues, seems to have an important role in the pathophysiology and presentation of this disease. In pancreas, this receptor is expressed in both exocrine glands and islets, being a potential target for the virus and subsequent pancreatic injury. There are few articles reporting pancreatic injury in COVID-19 patients but most of them do not report acute pancreatitis. Diagnosing acute pancreatitis secondary to SARS-CoV-2 infection is challenging due to the need to rule out other etiologies as well the notable heterogeneous presentations. Herein we report the case of a patient with COVID-19 who developed severe acute pancreatitis.
SARS-CoV-2 (severe acute respiratory syndrome coronavirus-2), the etiologic agent of COVID-19 pandemic, has spread rapidly worldwide since December 2019. Despite the infection primarily affecting the respiratory tract, gastrointestinal involvement has been reported in an increasing number of patients, with symptoms such as nausea, vomiting, diarrhea, abdominal pain and gastrointestinal bleeding.1 Laboratory abnormalities such as hepatic and pancreatic injury have been evident in a subset of patients, although it remains unclear if these abnormalities have any impact on prognosis.2,3
The more common causes of acute pancreatitis are gallstones and alcohol abuse, however viral-induced acute pancreatitis has also been described.4 Angiotensin-converting enzyme-2 (ACE2), the functional virus host cell receptor, expressed in both exocrine and endocrine pancreatic cells, plays a role in this disease process. The mechanisms of pancreatic injury in SARS-CoV-2 infection include direct cytopathic effects or indirect systemic inflammatory and immune-mediated cellular responses, resulting in organ damage or secondary enzyme abnormalities.1
This case report describes a patient with COVID-19 that developed severe acute pancreatitis.
Case reportA 56-year old female presented at the emergency department with dry cough, dyspnea, general malaise and epigastric pain which had persisted for a couple of days. Comorbidities included only hypertension treated with losartan and hydrochlorothiazide. The patient reported minimal alcohol intake and did not smoke.
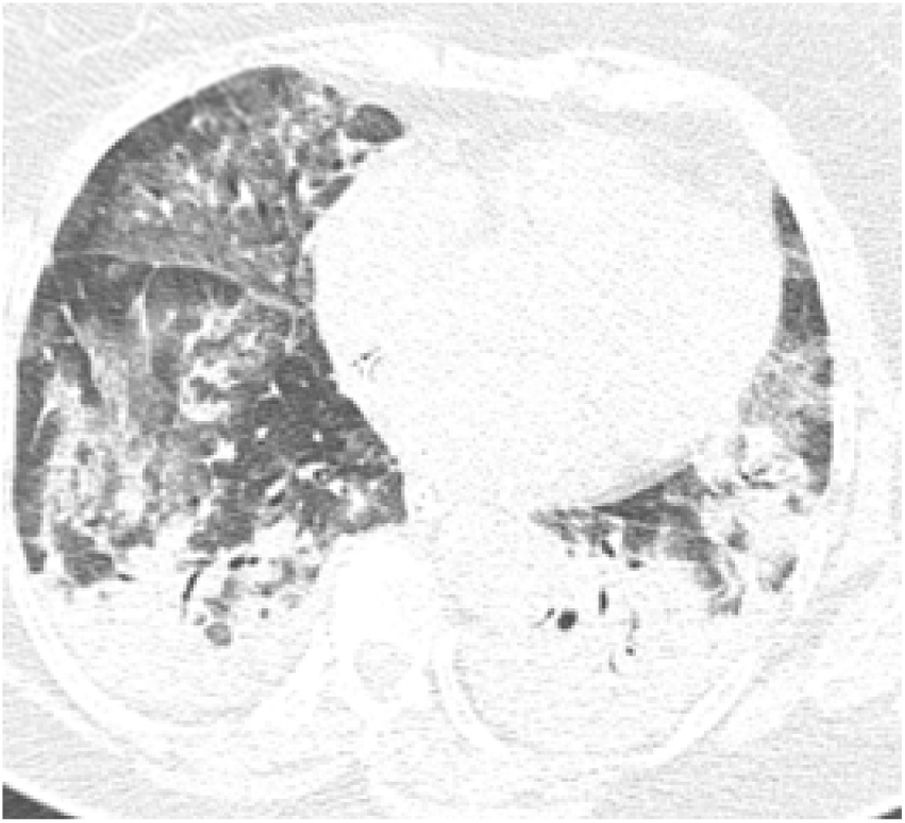
On initial examination, the patient was hemodynamically stable and presented only with tachypnea (24 breaths/min). Chest radiography showed diffuse interstitial opacities. She was admitted to an inpatient unit, but her condition worsened over the first seven days showing signs of acute respiratory distress, being transferred to the Intensive Care Unit and required mechanical ventilation for six days. She received intravenous antibiotics; however inotropic drugs were not indicated nor administered. COVID-19 was confirmed by Real Time-Polymerase Chain Reaction (RT-PCR) of a nasopharyngeal swab, and pulmonary computed tomography (CT) showed multiple ground-glass opacities, interlobular septal thickening and consolidation areas (Fig. 1).
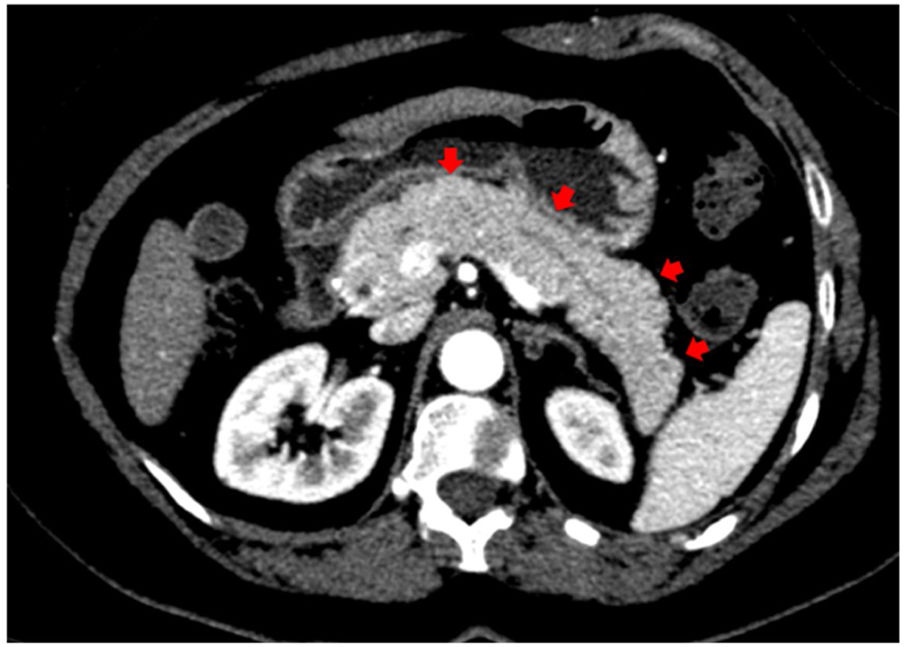
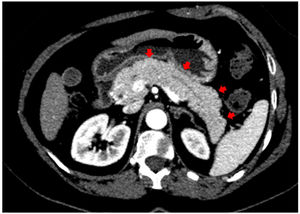
In addition to the pulmonary findings, chest CT showed pancreas abnormalities such as a tail parenchymal enlargement and surrounding retroperitoneal fat stranding (Fig. 2). The patient did not experience relevant abdominal symptoms throughout hospitalization, however, serum pancreatic enzymes were measured after the CT findings. The amylase and lipase level increased from 249 to 544 U/L and from 580 to 2993 U/L, respectively, over seven days. Her glucose level was varying between 121–182 mg/dL. A daily decline of amylase and lipase were observed afterwards. Although the Modified Glasgow Acute Pancreatitis Score was 3 points indicating severe acute pancreatitis, no complications were identified, and only supportive therapy was needed such as intravenous fluids and a bland diet.
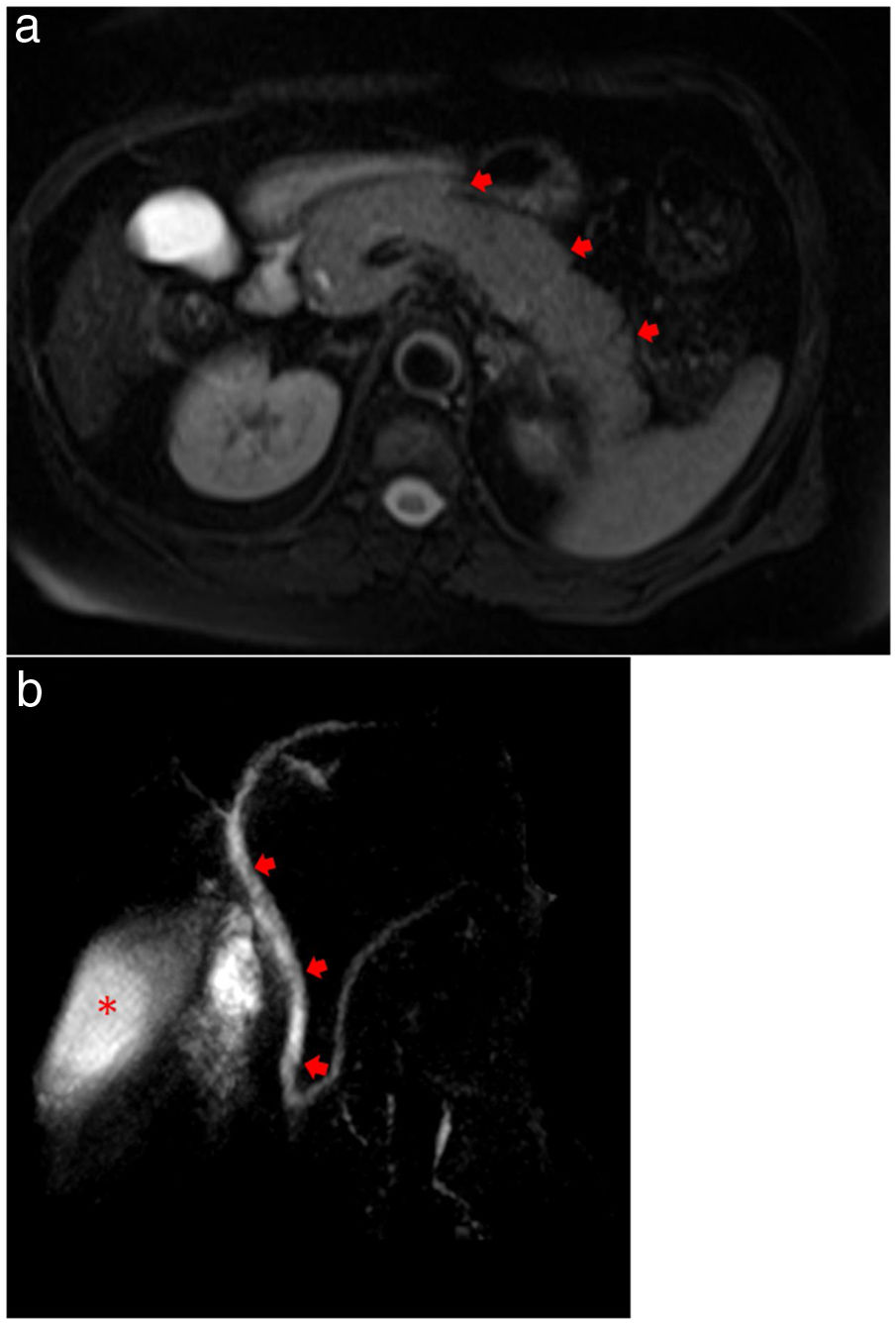
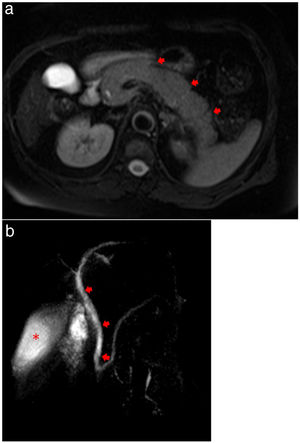
Magnetic resonance cholangiopancreatography (MRCP) showed evidence of acute pancreatitis with a diffusely enlarged pancreas without focal lesions or gallstones (Fig. 3a, b). Plasma level of triglycerides was 209 mg/dL and calcium level was normal (1.24 mg/dL). An Endoscopic Ultrasound was performed, after complete recovery from respiratory symptoms and after two negative results for RT-PCR, showing no microlithiasis.
(a) MRCP showing evidence of acute pancreatitis with a diffusely enlarged pancreas (arrows) without focal lesions or gallstones. (b) MRCP showing no extrahepatic biliary duct dilatation (arrows) and homogeneous contrast in gallbladder (asterisk), demonstrating no evidence of choledocholithiasis or cholelithiasis.
Other causes of acute pancreatitis such as drugs, trauma and hypotension were excluded, and the patient was discharged after 35 days of hospitalization without any long-term sequelae.
DiscussionInitially reported as a respiratory tract pathogen, SARS-CoV-2 has been identified in many other tissues, such as the cardiovascular, renal and gastrointestinal tract, similar to SARS-CoV in 2003.5,6 Both viruses have ACE2 as the functional host cell receptor, enabling virus entry and replication.1,5,7 However, different from SARS-CoV, SARSCoV-2 does not use other receptors such as aminopeptidase N and dipeptidyl peptidase 4,8 being more selective. Furthermore, SARS-CoV-2 has higher affinity to ACE2 when compared to SARS-CoV, being more pathogenic5 and increasing the ability of community transmission.9
ACE2 is abundantly expressed in many different tissues, justifying the involvement of different organs and extrapulmonary symptoms of those diseases.1,5,7 The expression of ACE2 in the gastrointestinal tract during SARS-CoV-2 infection leads to digestive system dysregulation.1 Symptoms like nausea, vomiting and diarrhea have commonly been described in 11%–50% of cases.10–12 Gastrointestinal findings are significant due to their association with adverse outcomes such a delayed hospital admission and evidence of more laboratory changes, including prolonged coagulation time.10
In addition to gastrointestinal symptoms, some blood abnormalities were found in severe patients, such as increased pancreatic enzymes,1,3,7,10 suggesting pancreatic injury. In spite of gallstones and alcohol abuse being reported as the more common causes of acute pancreatitis, infectious agents, especially virus, are responsible for approximately 10% of cases,5 such as mumps, cytomegalovirus and influenza.13,14 Therefore, it is likely to consider SARS-COV-2 as a potential cause of pancreatitis. Curiously, in a recent study published by Schepis et al., SARS-COV-2 RNA was detected in a pancreatic pseudocyst sample endorsing pancreatic involvement in COVID-19.15
Furthermore, the mRNA level of ACE2 in pancreas was shown to be higher than in lung and expressed in both the exocrine glands and islets, being potential targets of SARS-CoV-2, resulting in pancreatic injury.7 Although the density of ACE2 in pancreatic tissue is still controversial6,7,16 and has individual variation,7 higher mRNA ACE density, during those virus infections, may signal greater predisposition to trigger acute pancreatitis.16
ACE2 receptor is highly expressed in pancreatic islet cells,16 therefore SARS-CoV-2 infection can theoretically cause islet damage resulting in acute diabetes.7 The patient in this case presented increased blood glucose levels, as found in six of nine patients with pancreatic injury in another study.3 Dysglycemias were already observed with SARS-CoV16 and may alter disease prognosis, since diabetes and ambient hyperglycemia were independent predictors for death and morbidity in SARS patients.16,17 Fortunately, a minority of these patients progressed to diabetes three years after hospital discharge.16
Besides glucose changes due to SARS-CoV-2 affinity for pancreatic islets, acute pancreatitis may occur if the virus damages the exocrine cells. The mechanisms involve direct and indirect pancreatic injury. Direct cytopathic effects are mediated by the local SARS-CoV-2 replication itself, while indirect mechanisms involves an exacerbated systemic inflammatory response syndrome (SIRS) and immune-mediated cellular responses caused by own immune response induced by SARS-CoV-2 infection and the respiratory impairment caused by this virus.2,3
Two studies from China reported laboratory and imaging findings of pancreatic injury in COVID-19 patients admitted in separate hospitals. Both studies demonstrated significant pancreatic involvement in this disease, but failed to identify the association between those findings, as well as to report the presence of pancreatitis symptoms, making it difficult to establish an acute pancreatitis diagnosis. According to the current guidelines,18 diagnosis of acute pancreatitis requires at least two of the three following signs: (1) abdominal pain, (2) amylase or lipase >3 times the upper normal limit, and (3) characteristic findings on diagnostic imaging.
In the first study, conducted by Wang et al., nine out of 52 patients (17%) had pancreatic enzyme abnormalities, with any change above the upper limit of normality being considered, and six of them (66%) also had hyperglycemia. No imaging tests were described, nor whether any of the patients had criteria for acute pancreatitis. Patients with pancreatic injury had a higher incidence of gastrointestinal symptoms, such as diarrhea and anorexia, in addition to severe disease on admission. When compared with patients without pancreatic injury, there was no difference regarding mechanical ventilation or viral clearance.3
In the second study, Liu et al. analyzed 121 patients admitted into hospital, 64 (52.9%) diagnosed with a severe form of COVID-19. Of these patients, 12 (18%) had pancreatic enzyme abnormalities compared to one patient (1.85%) with non-severe COVID-19. Corroborating the findings of Wang et al., a strong association between severe disease and pancreatic injury was demonstrated. However, in six of those 13 patients, there was a report of anti-inflammatory drugs and glucocorticoids, which could have been responsible for the pancreatic injury. Regarding tomographic changes, 7.46% had some pancreatic finding. However, this article also did not describe whether any of these patients had criteria for acute pancreatitis, nor did report if serum values of amylase and lipase from those patients were associated with imaging changes.7
Those articles demonstrated how asymptomatic or mildly gastrointestinal symptomatic patients with COVID-19 and pancreatic enzymes abnormalities could be overlooked and acute pancreatitis underdiagnosed. Herein we presented a patient with acute pancreatitis suspected due to altered pancreatic enzymes, with little or no gastrointestinal symptoms, and with subsequent diagnosis confirmed by CT scans. Similar to this case, Anand et al. diagnosed acute pancreatitis in a COVID-19 patient by examining the CT scan, which was ordered due to suspected bowel obstruction.19 The notable heterogeneous presentation and the need to rule out other main etiologies, due to this rare association, are some of the challenges in the diagnosis.
ConclusionSARS-CoV-2 seems to have some tropism for pancreatic (exocrine and endocrine) cells, causing acute pancreatitis. Physicians should be aware that asymptomatic or mildly gastrointestinal symptomatic patients with COVID-19 require pancreatic enzymes and even abdomen imaging to diagnose pancreatitis. This diagnosis is important for adequate treatment and better management of systemic repercussions, such as SIRS, decreasing SARS-CoV-2 mortality.
FundingNo funding.
Conflicts of interestThe author declares no conflicts of interest.
Informed consentThis study was a case report study, patient identity remained anonymous, and the informed consent was obtained.
Authors’ contributionsF.J.C, A.C.S.T and M.A.N.M the concept and design of the study; A.M.A and E.Y.Y data acquisition and interpretation of the data; A.MA, E.Y.Y and M.A.N.M literature review, and drafted the manuscript. All authors critically revised the manuscript, approved the final version to be published, and agree to be accountable for all aspects of the work.
We thank Dr. Sergio Matuguma for performing the Ecoendoscopy exam. We thank Dr. Joao Marcos Wolf Maciel for providing the radiographic images. We thank Mr. Timothy Finian Coyne for reviewing the English version.