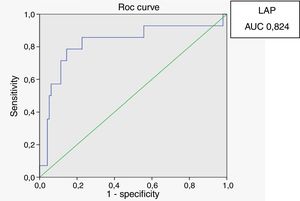
The lipid accumulation product (LAP) index is an emerging cardiovascular risk marker. We aimed to assess the accuracy of this index as a marker of cardiovascular risk in HIV-infected patients. A cross-sectional study of 133 HIV-infected patients on antiretroviral drugs and 20 non-infected controls was conducted at the outpatient clinic of a referral center of infectious and parasitic diseases. Evaluations included LAP index, homeostasis model assessment (HOMA) index, anthropometric measurements, blood pressure, glucose tolerance test, and cholesterol and triglyceride levels. Body mass index (BMI) was similar in both groups; however, waist circumference was greater in the HIV-infected patients. Triglyceride levels were significantly higher (p<0.001) and HDL cholesterol levels were lower in HIV-infected patients (p<0.001). Plasma glucose (p=0.01) and insulin (p=0.005) levels two hours after a glucose load, HOMA-IR index (p<0.001) and LAP index (p<0.001) were higher in the HIV-infected patients. A positive and significant correlation was found between HOMA-IR index and LAP (r=0.615; p<0.01), BMI (r=0.334; p<0.01) and waist circumference (r=0.452; p<0.01) in the HIV-infected patients. In male HIV-infected patients and controls, ROC curve analyses revealed that the best cut-off value of LAP to define the presence of insulin resistance was 64.8 (sensitivity 86%, specificity 77% and area under the curve 0.824). These results confirm that insulin resistance is more common in HIV-patients on antiretroviral drugs than in HIV-negative controls. A positive and significant correlation was found between the LAP index and the HOMA index, with LAP≥64.8 constituting an additional risk factor for cardiovascular disease in male HIV patients.
The introduction of highly-active antiretroviral therapy (HAART) has significantly improved the clinical outcome of individuals with the human immunodeficiency virus (HIV), resulting in increased survival rates1–3; however, the success of antiretroviral therapy is tempered by long-term side effects that include dyslipidemia, insulin resistance, overt type 2 diabetes mellitus, and changes in fat distribution (peripheral lipoatrophy and visceral adiposity).4–6
The pattern of these metabolic abnormalities in patients receiving antiretroviral therapy resembles that of the metabolic syndrome, which is known to increase the risk of cardiovascular disease. However, whether and how soon these antiretroviral therapy-induced abnormalities may result in a clinically detectable increased risk of cardiovascular disease remains unknown, as does the impact of the underlying HIV infection per se. Indeed, while results from long-term observational studies on the risk of cardiovascular disease in HIV-infected patients remain unavailable in the literature, calculating the predicted risk of cardiovascular disease may prove useful for the clinical management of these patients.
Insulin resistance is an independent risk factor for cardiovascular disease and the early recognition of insulin resistance in these patients is important for the prevention of cardiovascular involvement.
Euglycemic-hyperinsulinemic clamping is currently the gold standard for measuring insulin resistance. However, it is unsuitable for clinical practice, since it is complex, expensive and unfeasible for large populations.7 Taking into consideration the technical limitations of clamping and alternative methods (those that rely on the measurement of insulin itself) for identifying insulin resistance, some investigators have speculated that insulin resistance and, therefore, cardiovascular risk could be determined based on variables associated with the effects of insulin instead of measuring insulin directly.8,9
The lipid accumulation product (LAP) index, which is based on a combination of waist circumference and fasting triglyceride levels, could be useful in this situation. LAP is determined by the following equation for women: (circumference of waist [cm] – 58) × (triglycerides [mmol/L]); for men: [waist (cm) – 65] multiplied by triglyceride level (mmol/L). Results are expressed in cmmmol/L.10 Kahn10 was the first to describe LAP, reporting in the National Health and Nutrition Examination Survey (NHANES III) that LAP was a better indicator of cardiovascular risk in adults than body mass index (BMI).
Therefore, the aim of this study was to determine the accuracy of the LAP index as a marker of cardiovascular risk in HIV-infected patients and non-infected controls.
Patients and methodsDesign and patientsThis cross-sectional study was conducted at the outpatient clinic of a referral and training Center in Infectious and Parasitic Diseases in Brazil. The sample was selected for convenience. Sample size calculation of the case group assumed: (a) number of patients infected by HIV in 2013, age group 19–40 years, Belo Horizonte (n=157)11; (b) prevalence of lipodystrophy (84%)12; (c) variation of 5%; (d) confidence level equal to 95%. The minimum sample size was 90 individuals.
The case group comprised 133 HIV-infected patients aged between 18 and 55 years, who had been receiving antiretroviral (ARV) drugs for a minimum of three months prior to admission, regardless of the time of infection diagnosis. The control group consisted of 20 HIV-negative controls.
Exclusion criteria consisted of metabolic disorders such as hyperlipidemia, diabetes mellitus and lipodystrophy prior to the diagnosis of HIV-infection; use of glucocorticoids or any other steroids, growth hormones, beta-blockers, thiazides or any drugs associated with metabolic abnormalities and body fat redistribution. Other criteria included any relevant clinical event at the time of enrollment to the study; refusal to participate; pregnancy or breastfeeding; and alcohol abuse.
The study was approved by the COEP and conducted according to the norms of the code of ethics for human research, National Health Council, Resolution n° 466/2012 and all participants gave their written informed consent.
Study protocolAnthropometric data were measured according to the procedures standardized by the World Health Organization.13 To measure height, the stadiometer of the anthropometric scale of the brand “Filizzolla®” was used. Body weight was determined on an anthropometric scale of the Tanita brand with a capacity of 150kg. Waist circumference was defined as the smallest measurement at the midpoint between the lateral iliac crest and the lowest rib.14 BMI (weight in kg divided by height in meters squared) was calculated.
The skin folds were measured with the aid of a plicometer (Lange® caliper, Santa Cruz, CA, USA) with an accuracy of 0.1mm. Bicipital (DCB) and triceps skinfolds were measured in the anterior and posterior region. The subscapular skinfold (DCSE) was measured obliquely in relation to the longitudinal axis, following the orientation of the costal arcs, being located two centimeters below the angle. The supra-iliac cutaneous fold was obtained obliquely in relation to the longitudinal axis, in the mid point between the last costal arch and the iliac crest, on the median axillary line.15
Blood pressure was measured in the right arm, at the level of the heart, using a Welch Allyn Tycos® sphygmomanometer, model 705014 (New York, NY, USA). Values above 140×90mmHg were considered high.
The subjects were asked about the practice, type, duration and frequency of physical activity (FA) being classified as very active (FA >5 days/week and ≥30min/session), active (FA 3 or 4 days/week and ≥20min/session), not very active (those individuals who perform physical activity, but insufficient to be classified as active because it does not comply with the recommendations regarding frequency or duration), and sedentary (those who do not perform any physical activity).16
AssaysBlood samples were taken after a 12-h overnight fast and two hours after a 75g oral glucose load. Plasma glucose, total cholesterol and triglycerides were determined enzymatically (Vitros Chemistry Products, Johnson & Johnson Clinical Diagnostics®, Rochester, USA). HDL-cholesterol was measured using an immuneinhibition assay (Labtest Diagnosis®, Lagoa Santa, Brazil), and insulin was measured by chemiluminescence (Diagnostic Products Corporation®, Los Angeles, CA, USA). LDL-cholesterol was calculated. LDL was not evaluated in patients with triglyceride levels above 400mg/dl.
Insulin resistance was estimated using the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) based on the following equation: fasting insulin level in μU/mL multiplied by fasting glucose level in mmol/L divided by 22.5.17 The cutoff point to define IR was arbitrarily defined as a HOMA index ≥ 2.71.18 The LAP index was calculated using the formula [waist (cm) – 58] multiplied by triglyceride level (mmol/L) for women and [waist (cm) – 65] multiplied by triglyceride level (mmol/L) for men.10
Statistical analysisThe data were stored using double-key punching with software Epidata, version 3.1. Quantitative variables are presented as means ± standard deviations of the mean or medians, according to their distribution assessed by the Shapiro–Wilk test. A variable was considered as having a normal distribution if p<0.05 and as non-parametric if p>0.05. Categorical variables are presented as percentages.
The chi-square test was performed to compare dichotomous variables, while differences between the mean (or median) values of continuous variables were assessed using Student's t-test or the Mann–Whitney test, the latter being used in the case of variables with non-Gaussian distribution.
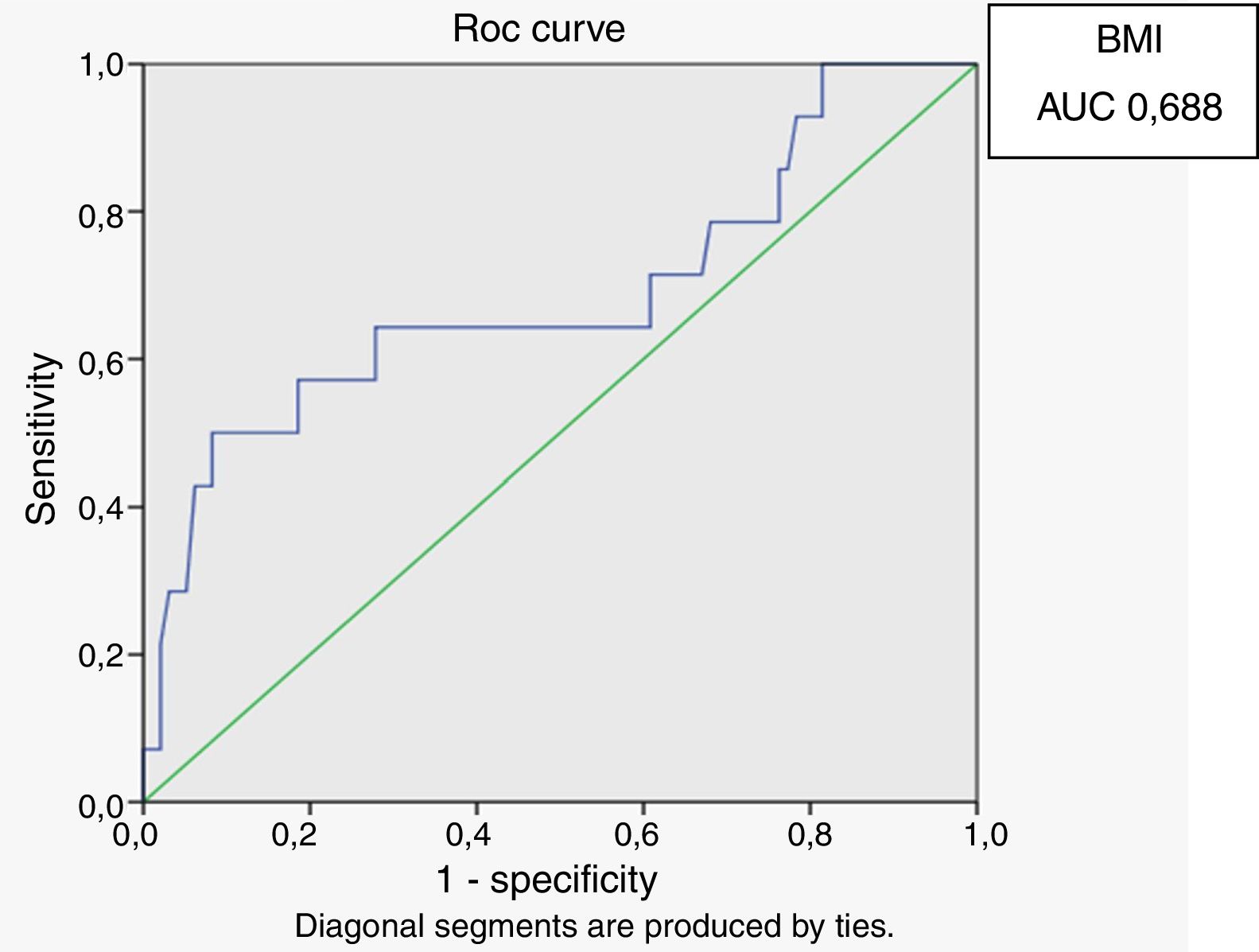
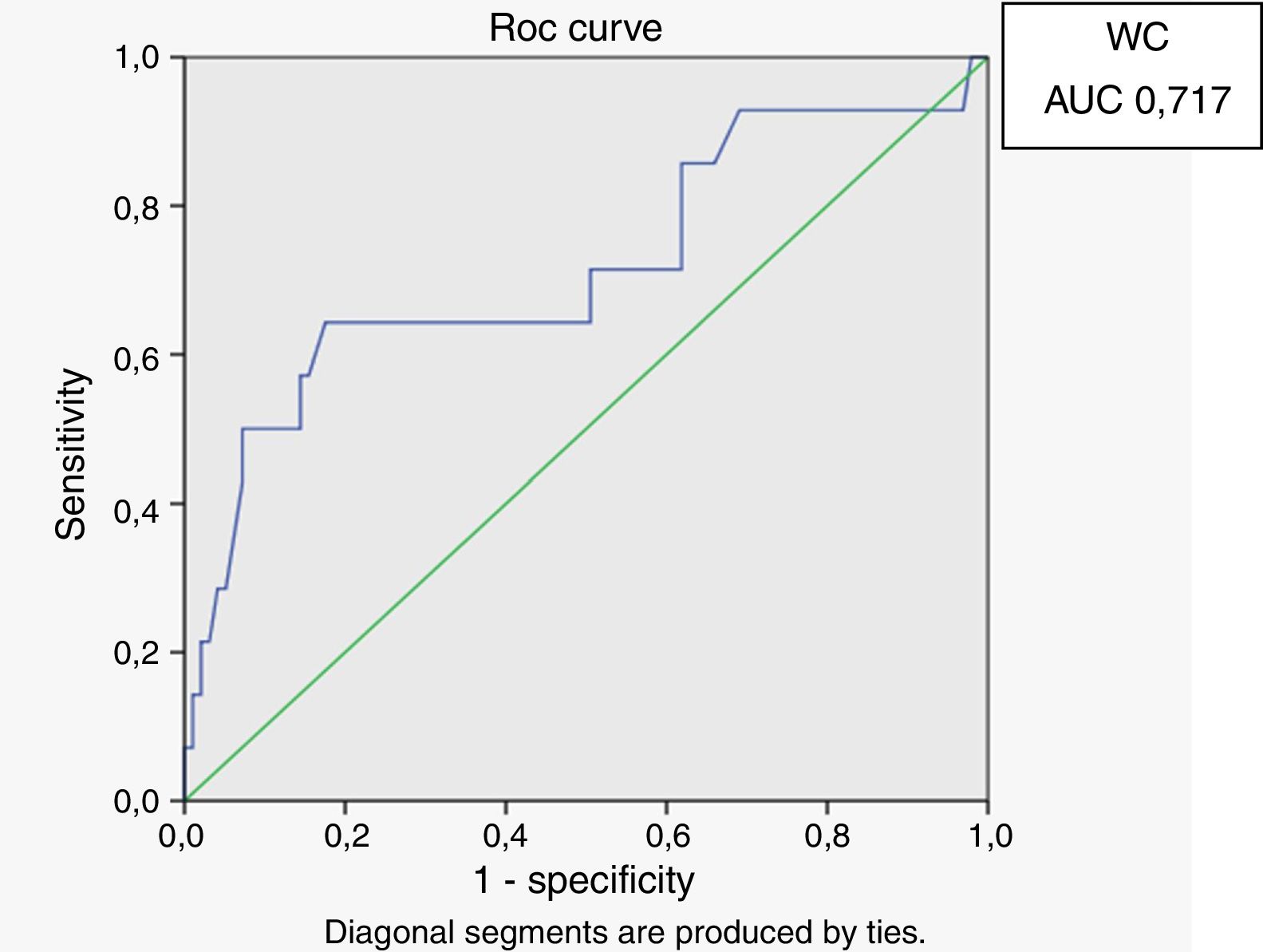
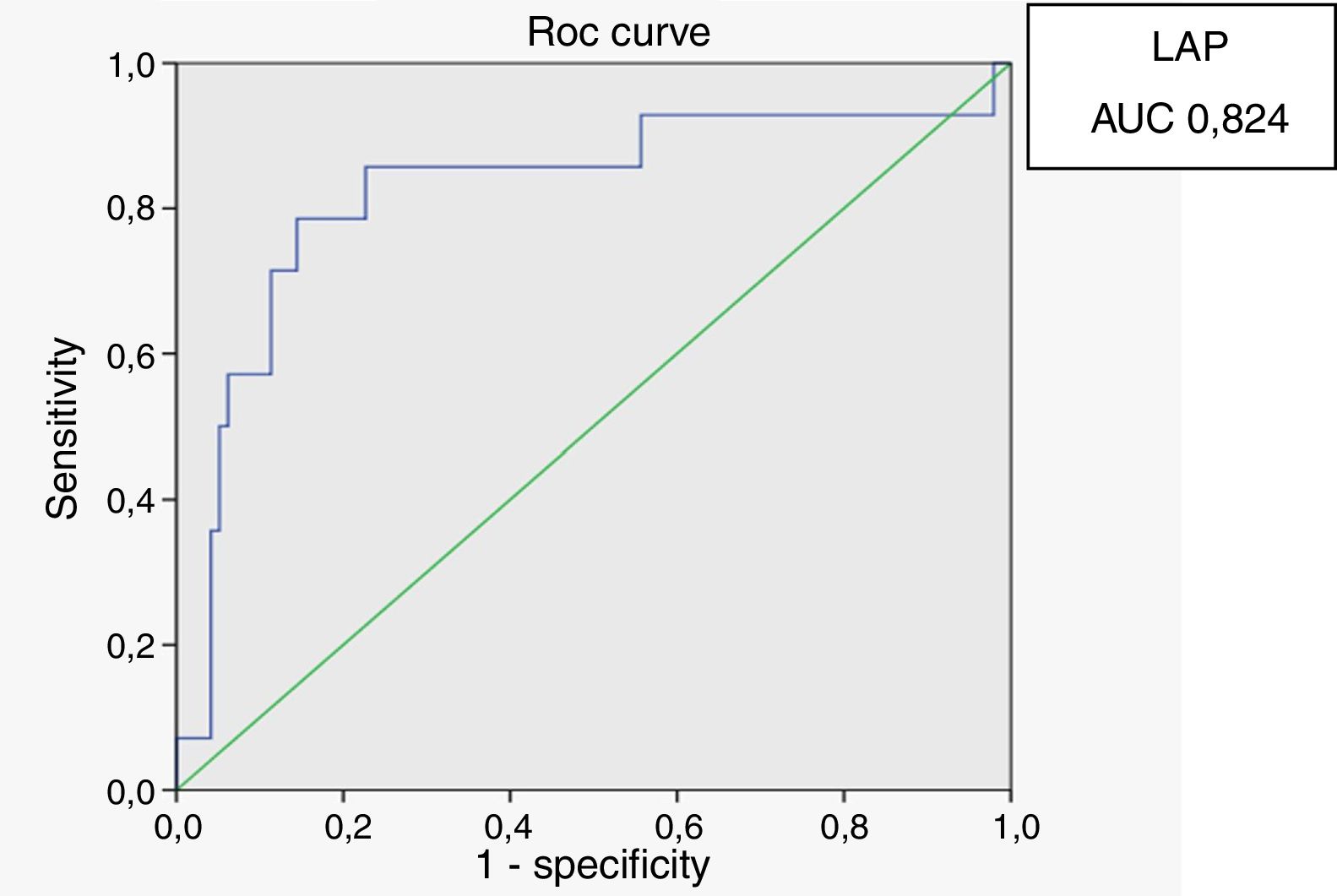
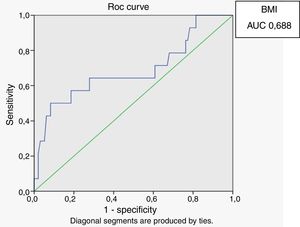
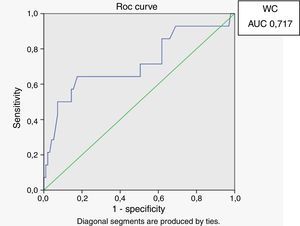
Receiver operating characteristic (ROC) curves were generated for the LAP index, BMI and waist circumference, with a HOMA-IR index of 2.71 as a marker of insulin resistance. Sensitivity and specificity for LAP were calculated based on the point of inflection in the ROC curve. Sensitivity and specificity for BMI, waist circumference and HOMA index were calculated using validated cutoffs (25 for BMI and 94 for waist circumference). Since there are gender differences in the validated cut-off levels for waist circumference, the ROC curves were generated only for HIV-infected men.
The non-parametric Spearman rank correlation coefficient was used to assess agreement between LAP and the continuous independent variables. A two-tailed p-value <0.05 was considered statistically significant. The classification suggested by Landis and Koch19 was used as follows: poor-to-fair agreement (kappa <0.40), moderate agreement (kappa of 0.41 to 0.60), substantial agreement (kappa of 0.61 to 0.80), and excellent agreement (kappa of 0.81 to 1.0). A two-tailed p-value <0.05 was considered statistically significant.
All statistical analyses were performed using SPSS, version 18.0 for Windows (Chicago, IL, USA).
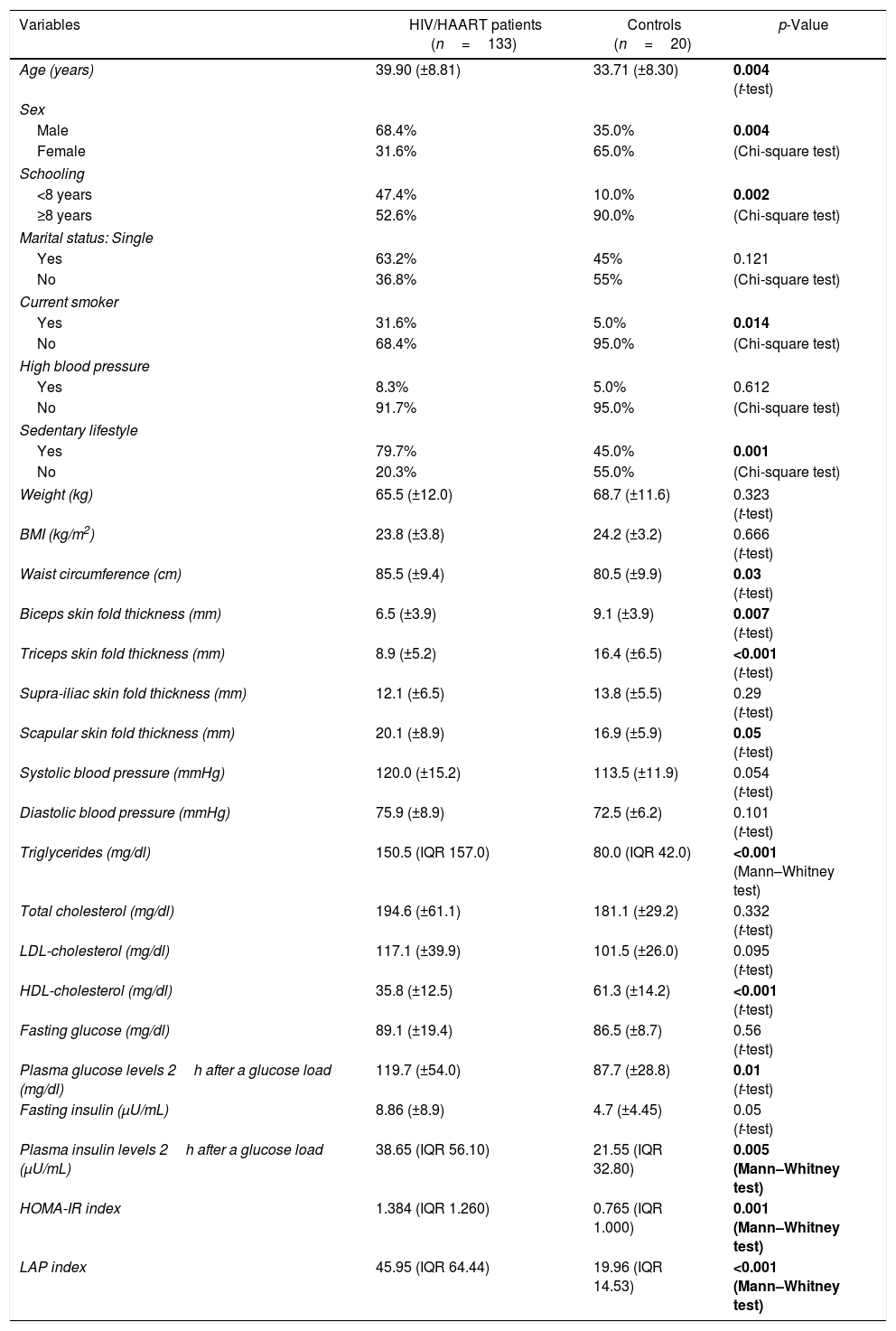
ResultsThe study included 133 HIV-infected patients and 20 non-infected individuals. There were no statistically significant differences between the HIV-infected patients and the control group in relation to their family history of diabetes mellitus, stroke, coronary artery disease, dyslipidemia or arterial hypertension. Table 1 summarizes the clinical and metabolic profiles of the two groups.
Clinical and metabolic features of HIV-infected patients on highly-active antiretroviral therapy (HAART) and non-infected controls.
| Variables | HIV/HAART patients (n=133) | Controls (n=20) | p-Value |
|---|---|---|---|
| Age (years) | 39.90 (±8.81) | 33.71 (±8.30) | 0.004 (t-test) |
| Sex | |||
| Male | 68.4% | 35.0% | 0.004 |
| Female | 31.6% | 65.0% | (Chi-square test) |
| Schooling | |||
| <8 years | 47.4% | 10.0% | 0.002 |
| ≥8 years | 52.6% | 90.0% | (Chi-square test) |
| Marital status: Single | |||
| Yes | 63.2% | 45% | 0.121 |
| No | 36.8% | 55% | (Chi-square test) |
| Current smoker | |||
| Yes | 31.6% | 5.0% | 0.014 |
| No | 68.4% | 95.0% | (Chi-square test) |
| High blood pressure | |||
| Yes | 8.3% | 5.0% | 0.612 |
| No | 91.7% | 95.0% | (Chi-square test) |
| Sedentary lifestyle | |||
| Yes | 79.7% | 45.0% | 0.001 |
| No | 20.3% | 55.0% | (Chi-square test) |
| Weight (kg) | 65.5 (±12.0) | 68.7 (±11.6) | 0.323 (t-test) |
| BMI (kg/m2) | 23.8 (±3.8) | 24.2 (±3.2) | 0.666 (t-test) |
| Waist circumference (cm) | 85.5 (±9.4) | 80.5 (±9.9) | 0.03 (t-test) |
| Biceps skin fold thickness (mm) | 6.5 (±3.9) | 9.1 (±3.9) | 0.007 (t-test) |
| Triceps skin fold thickness (mm) | 8.9 (±5.2) | 16.4 (±6.5) | <0.001 (t-test) |
| Supra-iliac skin fold thickness (mm) | 12.1 (±6.5) | 13.8 (±5.5) | 0.29 (t-test) |
| Scapular skin fold thickness (mm) | 20.1 (±8.9) | 16.9 (±5.9) | 0.05 (t-test) |
| Systolic blood pressure (mmHg) | 120.0 (±15.2) | 113.5 (±11.9) | 0.054 (t-test) |
| Diastolic blood pressure (mmHg) | 75.9 (±8.9) | 72.5 (±6.2) | 0.101 (t-test) |
| Triglycerides (mg/dl) | 150.5 (IQR 157.0) | 80.0 (IQR 42.0) | <0.001 (Mann–Whitney test) |
| Total cholesterol (mg/dl) | 194.6 (±61.1) | 181.1 (±29.2) | 0.332 (t-test) |
| LDL-cholesterol (mg/dl) | 117.1 (±39.9) | 101.5 (±26.0) | 0.095 (t-test) |
| HDL-cholesterol (mg/dl) | 35.8 (±12.5) | 61.3 (±14.2) | <0.001 (t-test) |
| Fasting glucose (mg/dl) | 89.1 (±19.4) | 86.5 (±8.7) | 0.56 (t-test) |
| Plasma glucose levels 2h after a glucose load (mg/dl) | 119.7 (±54.0) | 87.7 (±28.8) | 0.01 (t-test) |
| Fasting insulin (μU/mL) | 8.86 (±8.9) | 4.7 (±4.45) | 0.05 (t-test) |
| Plasma insulin levels 2h after a glucose load (μU/mL) | 38.65 (IQR 56.10) | 21.55 (IQR 32.80) | 0.005 (Mann–Whitney test) |
| HOMA-IR index | 1.384 (IQR 1.260) | 0.765 (IQR 1.000) | 0.001 (Mann–Whitney test) |
| LAP index | 45.95 (IQR 64.44) | 19.96 (IQR 14.53) | <0.001 (Mann–Whitney test) |
Bold value indicates a p-value less than 0.05.
BMI was similar in both groups; however, the HIV-infected patients had a greater waist circumference. Biceps and triceps skin fold thickness was significantly thinner and scapular skin fold thickness was greater in the HIV-infected patients compared to the controls. Triglyceride levels were significantly higher and HDL cholesterol levels were lower in the HIV-infected group. Plasma glucose and insulin levels two hours after a glucose load, HOMA-IR index, and LAP index were significantly higher in the HIV-infected patients.
A positive and significant correlation was found between the HOMA index and LAP, BMI and waist circumference in HIV-infected patients (Table 2).
Spearman's rank correlation coefficient between HOMA index and lipid accumulation product (LAP), body mass index, and waist circumference.
| Independent variables | Spearman's rank correlation coefficient (r) | p-Value |
|---|---|---|
| Lipid accumulation product | 0.615 | <0.01 |
| Body mass index | 0.334 | <0.01 |
| Waist circumference | 0.452 | <0.01 |
In male HIV-infected patients, analysis of the ROC curve revealed that the best cutoff value for LAP to define the presence of insulin resistance was 64.8 (sensitivity 86%, specificity 77% and area under the curve [AUC] 0.824). ROC curves were also generated for BMI and waist circumference (WC) using the cutoffs of 25 for BMI (sensitivity 57%, specificity 80% and AUC 0.688) and 94cm for WC (sensitivity 50%, specificity 87% and AUC 0.717) (Figs. 1–3).
In the present study, the LAP index was investigated for the first time in HIV-infected patients. LAP indexes were found to be higher in HIV-infected patients compared to controls, suggesting increased insulin resistance and cardiovascular risk in HIV-infected patients on antiretroviral therapy. Over recent decades, it has become clear that HIV-infected patients on antiretrovirals develop metabolic disturbances that resemble those found with the metabolic syndrome4–6 and, moreover, several cohort studies have reported a higher risk of myocardial infarction in HIV-infected patients.20,21
Identifying insulin resistance in clinical practice remains a challenge. The gold standard, euglycemic hyperinsulinemic clamping is clearly inadequate in view of its high cost and complexity. Alternative methods that use fasting insulin levels can lead to a misdiagnosis of insulin resistance, since there is no well-established reference range for normal insulin levels.22 For this reason, indexes that correlate well with the clamp and depend on fasting insulin, such as the HOMA-IR index, are difficult to implement as a clinical test.
The LAP index is based on a combination of WC measurement and fasting triglyceride levels.10 It was developed to reflect the combined anatomical and physiological changes associated with lipid over-accumulation in adults. In that study, Kahn compared the LAP index to BMI in terms of the ability to identify cardiovascular risk in adults, using data from the NHANES III. Compared to BMI, correlations between the LAP index and lipid risk variables were stronger, suggesting that LAP might be a better predictor of the incidence of cardiovascular disease.
Previous studies have evaluated the LAP index in different insulin-resistant populations such as individuals with type 2 diabetes mellitus23 and polycystic ovary syndrome (PCOS)24,25; however, this is the first time that the LAP index has been evaluated in HIV patients. Results from the present study show a strong correlation between the LAP index and the HOMA-IR index in HIV-infected patient. In our opinion, screening this population that is prone to developing insulin resistance-related comorbidities, including cardiovascular diseases, prior to the onset of symptoms may prove useful.
It is interesting to note that although BMI was similar in the two groups, WC was greater in the HIV-infected patients. In addition, total cholesterol and LDL cholesterol levels were similar in the two groups; however, triglyceride levels were significantly higher in the HIV-infected patients. Previous studies have evaluated increased WC and high triglyceride levels in different populations as a surrogate marker of cardiovascular risk, with results showing that these two variables were more sensitive than the metabolic syndrome itself as markers of greater cardiovascular risk.26,27
According to the ROC curve, with a LAP index ≥ 64.8, sensitivity (86%) and specificity (77%) were adequate for detecting a state of insulin resistance and performed better than BMI at a cutoff point of 25 and WC at a cutoff point of 94cm.
Some limitations of this study must be mentioned, principally with respect to the small sample size of control group; thus, the finding of higher LAP levels in HIV-infected patients compared to controls has to be interpreted with caution. However, this finding is in line with the results of other studies that showed an increased metabolic risk in this population. Another limitation of the present study is that euglycemic hyperinsulinemic clamping was not performed because of the technical difficulties and high cost. Nonetheless, there is a strong correlation between the HOMA IR index, used as a reference standard, and euglycemic hyperinsulinemic clamp results.28
In summary, the LAP index may be considered a reliable marker of insulin resistance and a risk factor to cardiovascular disease in male HIV-infected patients on antiretroviral therapy. Early recognition of patients who are prone to developing insulin resistance and related metabolic disturbances will allow therapeutic interventions to be implemented to reduce future cardiovascular risk.
Conflicts of interestThe authors declare no conflicts of interest.