Vaccination against the hepatitis A virus (HAV) administered in two doses has been used effectively in universal child immunization programs in several countries. A single-dose vaccination was adopted in some low-income countries in an attempt to reduce costs without losing effectiveness. In 2014, single-dose universal vaccination was introduced in Brazil for children aged two years. Since such strategy is still not universally accepted, its efficacy should be compared to the two-dose strategy. To assess the humoral response after the single-dose HAV vaccination schedule, a cross-sectional study was conducted in Primavera do Leste, in Mato Grosso state, Central Brazil, including 265 children vaccinated through the National Immunization Program. Blood was collected by using a digital puncture and further applied to filter paper cards. Anti-HAV was detected in 218 out of 265 dried blood spots (DBS). Blood venous samples were collected from 34 out of 47 children who were not anti-HAV positive in DBS samples. Eighteen of them tested positive for anti-HAV, giving a final score of 93.6% (236/252) of seropositivity. In conclusion, this study demonstrated a high rate of anti-HAV positivity in the short term after single-dose hepatitis A vaccination in the population investigated. Moreover, the DBS was shown to be a reliable tool for detecting anti-HAV antibodies.
Hepatitis A is an enterically transmitted virus, and infection is common worldwide, particularly in low-income countries and poor health conditions.1 In these regions, there are high levels of circulating hepatitis A virus (HAV), infecting the majority of the population during the first decade of life. The World Health Organization estimates that more than 200 million infections and 30,000 deaths occurred in 2005.2 The infection is usually benign and even asymptomatic in childhood; however, a small number of cases develop acute liver failure and this may result in death. In low-income countries, there is lower intensity exposure to HAV and the age of infection may be delayed to adulthood.3 In these groups, symptomatic or more serious infections are more common. Countries that improve the sanitary conditions of their populations usually show a change in the epidemiological pattern from high to intermediate endemicity. This phenomenon has been described in several countries, including Brazil.4,5
In addition to improving sanitary conditions, universal immunization of children is an important strategy to control the spread of HAV and reduce disease incidence.6 Vaccines against HAV have been available since the 1990s and are generally based on the inactivated virus.7 Two doses of the vaccine are recommended, the second dose being administered six to 18 months following the first dose. The vaccine is highly immunogenic, and almost all vaccinated healthy subjects develop antibodies. On the other hand, the costs of the vaccine are significant, and it was initially meant to be used in high-risk groups, such as health professionals, homosexuals, and persons traveling to areas of high endemicity.2 However, this strategy has not been successful in decreasing incidence rates of HAV infection. Conversely, countries implementing universal immunization for children aged two to five years, such as Israel and the United States, have reported a dramatic decrease in incidence of infection, with herd immunity extending to older population groups.8,9
In Latin America, Mayorga et al. followed up 105 Nicaraguan children immunized with a single dose of HAV vaccine.10 After 7.5 years HAV infection was documented in only one child, which corresponded to an estimated protective effect of 98%. In 2005, Argentina implemented a universal immunization program, providing a single-dose vaccine to children aged 12 months.11 The single-dose strategy took into account the high costs of the vaccine and the development of antibodies by over 90% of vaccinated persons soon after the first dose. Studies assessing the impact of this strategy have shown a substantial fall in incidence of HAV infection.12
Brazil has also experienced a period of epidemiological transition of HAV.13 In 2014, the Ministry of Health of Brazil introduced a similar vaccination program against HAV, based on a single-dose vaccine (document available in http://www.brasil.gov.br/saude/2014/07/vacina-contra-hepatite-a-passa-a-ser-oferecida-pelo-sus; 2016, extracted on 14.03.16). Children aged between 15 and 24 months were targeted by the immunization program.
The single-dose schedule may be a solution for many countries with limited resources. Therefore, it is crucial to assess these programs in countries where they have been implemented. Specifically, it is important to assess whether protection will last and whether these vaccination programs have an impact on the endemic levels of infection.14,15
This study evaluated the single-dose schedule of anti-HAV vaccination adopted by the Brazilian government in a small city in the state of Mato Grosso, Central Brazil. The aim was to estimate the prevalence of anti-HAV antibodies among children who had been vaccinated at least 30 days before.
Materials and methodsArea of studyA cross-sectional study was conducted in Primavera do Leste, in the state of Mato Grosso, located 234km east of the state capital, with a population of 58,370 inhabitants in 2014. It is a young but flourishing city, particularly in the agriculture-related sector, with a high human development index (HDI: 0752) and a low infant mortality rate (14.5/1000 live births in 2010, according Brazilian Institute of Geography and Statistics (http://cidades.ibge.gov.br/xtras/perfil.php?codmun=510704; 2016).
Inclusion of volunteers and collection of samplesBetween August 2014 and April 2015, a total of 1135 children in their second year of life received a single intramuscular dose of inactivated virus vaccine against monovalent HAV (Vaqta™ Ped/Adol, Merck Sharp & Dohme), containing approximately 25U of HAV antigen.
The sample size calculation was based on the number of children vaccinated (1135), an estimated 90% serocoversion rate of anti-HAV antibodies with variance of ±5%, and 95% confidence interval (CI). According to these parameters, 124 individuals had to be included. We decided to double the sample in order to counterbalance the impossibility of randomizing capturing volunteers.
Using the records of the municipal health authorities, families of children vaccinated at least 30 days previously were notified about the study by community health agents. The parents or guardians were requested to attend the primary care units on specific days in order to meet the investigators. The research protocol was discussed with parents and guardians and an informed consent form was required to be signed in order to include each child in the study. Parents and guardians answered questions regarding the demographics and health background of their children.
In order to simplify blood sample collection and minimize the reluctance of the parents and children to provide blood samples, capillary blood samples were collected by finger stick using appropriate devices and blood drops were applied to Whatman Protein Saver 903 Filter Paper Card until filling a preprinted circle of 12.7mm diameter.
Reassessment of children with negative testsIndividuals who tested negative in the DBS sample analysis were approached again for collection of blood from the antecubital vein, using the same commercial kit. Samples (DBS or antecubital vein blood) were considered positive if antibody concentration was greater than 20mIU/mL, according to the manufacturer's recommendation.
A second dose of the vaccine was provided to participants who tested negative in the two tests.
Laboratory phaseDried blood spots (DBS) were eluted following the procedures described by Melgaço et al. for anti-HAV detection in DBS samples.16 They were frayed and individually inserted in centrifuge micro-vials with 350μL of PBS T/BSA buffer solution (containing 0.2% Tween 20 and 5% BSA).
After agitation of the micro-vials for 30min, samples were kept for 16–20h at a temperature between +4 and +8°C, followed by new agitation for 30min. Filter papers were removed, and DBS eluates were stored at −20°C until tested.
The eluates and serum samples were analyzed by enzymatic immunoassay (EIA) for qualitative/quantitative determination of total antibodies to HAV based on competitive assay (ETI-AB-HAVK PLUS® N0136, DiaSorin, Saluggia, Italy). The laboratory procedures followed the manufacturer's recommendation. Briefly, 50μL of the samples, calibrator and controls were incubated with 50μL of neutralization solution containing HAV and 50μL of buffer solution in respective wells. The excess sample, free HAV and soluble complexes between HAV and anti-HAV were removed by one wash cycle and the enzyme conjugate was added to the wells and allowed to incubate. The excess enzyme conjugate was removed by one wash cycle and a chromogen/substrate solution was added to the wells and allowed to incubate. The intensity of staining, as measured by a spectrophotometer, indicates inversely proportional to the presence of anti-HAV antibodies. Absorbance value readings for samples are compared to a threshold value determined based on the mean absorbance of the calibrator.
To correct dilution generated during elution, the concentration values obtained from the eluates were multiplied by a factor considering dilution, as recommended by the manufacturer. The volume of blood absorbed on the DBS disk was calculated to be approximately 60μL. The average hematocrit of children in the study population was 37% and the serum amounted to 63% of blood obtained (37.8μL) on average. A 350μL of buffer solution for elution and a 1:2 dilution was used for testing. Therefore, the final eluate was approximately 9.2 times more diluted than serum samples from venous blood. This value was applied to compensate the eluate dilution in relation to the standard procedure used for plasma or serum.
Statistical analysis and ethical aspectsFor the purposes of the analysis, all individuals who tested positive for anti-HAV in DBS eluates or venous blood were considered to have a positive result due to action of the vaccine as few children in the second or third year of life would have already been exposed to HAV.
The data were stored in an electronic database created using the EpiData Entry 3.1 software and investigation was performed using EpiData Analysis 2.2 (EpiData Association, Denmark, 2008). Appropriate statistical tests were performed to compare categorical and continuous variables. We calculated the odds ratios (OR) and respective 95% CIs to assess potential associations of independent variables with positive results for anti-HAV antibodies. Independent variables were considered to be associated with positivity for anti-HAV, where the chance of random error was less than 5%. We constructed logistic regression models to adjust for associations found in the crude analysis using the Stata 6.0 software (Statacorp, College Station, USA, 1999).
The research protocol was registered in Plataforma Brazil, the Brazilian system for evaluating ethics on research involving human beings, under CAAE #42987015.0.0000.554. This system is managed by the National Health Council of Brazil. The protocol was approved by the Research Ethics Committee of the University Hospital Júlio Müller/Federal University of Mato Grosso, on 21 May 2015.
ResultsA total of 277 children were approached for the study. Of these, 12 children were not included for having been vaccinated in private clinics, immunized less than 30 days before being approached, or received a second dose of the vaccine. Out of the remaining 265 subjects, 218 (82.3%) were anti-HAV positive in DBS eluates.
Anti-HAV was further investigated in venous blood samples obtained from 34 out of 47 children who were anti-HAV negative in DBS eluates. Retest was not performed in the remaining 13 DBS-negative children due to parent refusal to consent to another blood sample collection. Therefore, the final study sample consisted of 252 children. Of these, 128 (50.8%) were male, with a mean age of 17 (SD=5) months. The majority of participants lived in urban area of the municipality and 80.4% of them had been vaccinated less than one year prior to the study. The mean elapsed time between vaccination and blood collection was 261.6 (SD=127.3) days. A total of 236 children (93.6%; 95% CI=90.1–96.2%) out of 252 tested anti-HAV positive. Of these, 218 were anti-HAV positive in DBS eluates and 18 were DBS-negative but tested positive in venous blood samples.
Ten children who were anti-HAV positive in DBS eluates had their EIA optical density very close to the cutoff. They were approached again for an antecubital venous blood sample. Nine had the positivity confirmed. In the only case that anti-HAV was not confirmed, the optic density was again very close to the cutoff.
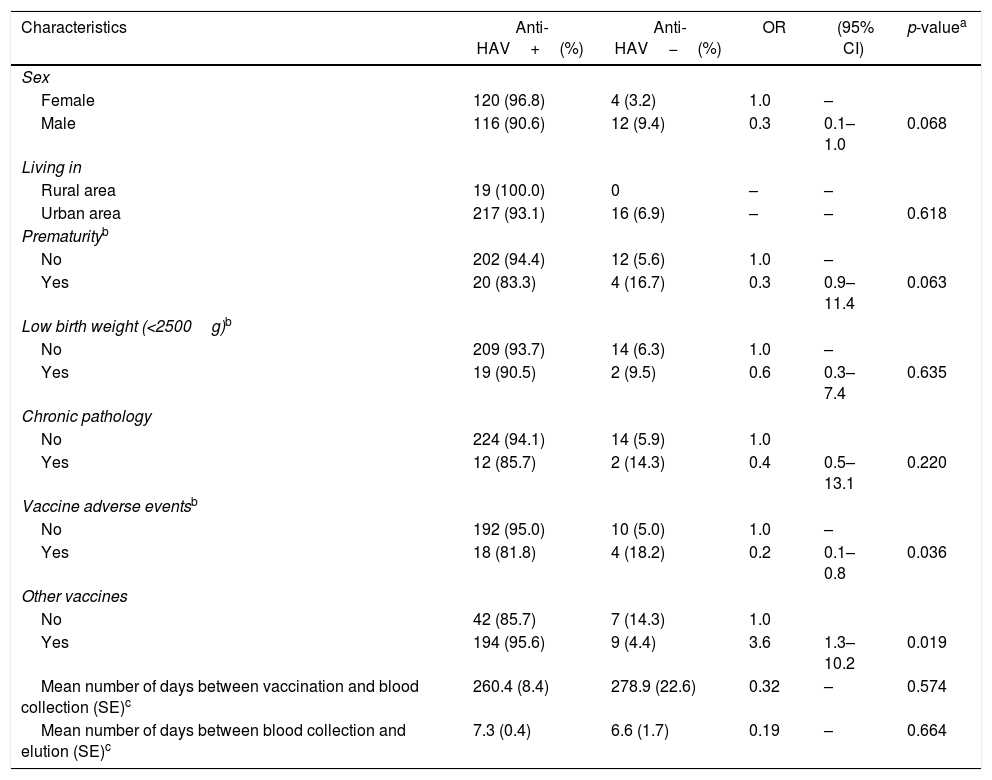
There was a higher rate of antibody response among female (96.8%) than in male (90.6%) participants, although this difference was not statistically significant (p=0.068). Age and living place (urban/rural) were not associated with anti-HAV status. Furthermore, no association was found between anti-HAV positivity and variables of interest, such as prematurity, low birth weight, comorbidities, and time between DBS blood sample collection and elution. Few participants (9.8%) reported mild vaccine-associated adverse events (fever, pain or local redness). These individuals had lower anti-HAV rate (81.8%) than participants who did not report these events (95.0%); this finding was statistically significant (p=0.036). There was also an association between presence of anti-HAV with concomitant administration of other vaccines on the same day of HAV vaccination (95.6%) compared to participants who received only the HAV vaccine (85.7%; p=0.019) (Table 1).
Association between selected factors and presence of anti-HAV in children vaccinated against HAV.
| Characteristics | Anti-HAV+(%) | Anti-HAV−(%) | OR | (95% CI) | p-valuea |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 120 (96.8) | 4 (3.2) | 1.0 | – | |
| Male | 116 (90.6) | 12 (9.4) | 0.3 | 0.1–1.0 | 0.068 |
| Living in | |||||
| Rural area | 19 (100.0) | 0 | – | – | |
| Urban area | 217 (93.1) | 16 (6.9) | – | – | 0.618 |
| Prematurityb | |||||
| No | 202 (94.4) | 12 (5.6) | 1.0 | – | |
| Yes | 20 (83.3) | 4 (16.7) | 0.3 | 0.9–11.4 | 0.063 |
| Low birth weight (<2500g)b | |||||
| No | 209 (93.7) | 14 (6.3) | 1.0 | – | |
| Yes | 19 (90.5) | 2 (9.5) | 0.6 | 0.3–7.4 | 0.635 |
| Chronic pathology | |||||
| No | 224 (94.1) | 14 (5.9) | 1.0 | ||
| Yes | 12 (85.7) | 2 (14.3) | 0.4 | 0.5–13.1 | 0.220 |
| Vaccine adverse eventsb | |||||
| No | 192 (95.0) | 10 (5.0) | 1.0 | – | |
| Yes | 18 (81.8) | 4 (18.2) | 0.2 | 0.1–0.8 | 0.036 |
| Other vaccines | |||||
| No | 42 (85.7) | 7 (14.3) | 1.0 | ||
| Yes | 194 (95.6) | 9 (4.4) | 3.6 | 1.3–10.2 | 0.019 |
| Mean number of days between vaccination and blood collection (SE)c | 260.4 (8.4) | 278.9 (22.6) | 0.32 | – | 0.574 |
| Mean number of days between blood collection and elution (SE)c | 7.3 (0.4) | 6.6 (1.7) | 0.19 | – | 0.664 |
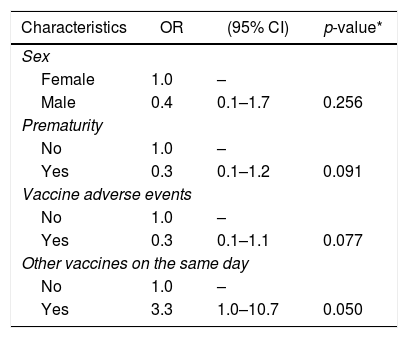
The associations identified in univariate analysis were adjusted by multiple logistic regression, including variables with p<0.2. The final model included 210 individuals. The presence of anti-HAV was associated only with having received another vaccine concurrently (p=0.050) (Table 2).
Multiple logistic regression analysis including variables associated with anti-HAV (p<0.2).
| Characteristics | OR | (95% CI) | p-value* |
|---|---|---|---|
| Sex | |||
| Female | 1.0 | – | |
| Male | 0.4 | 0.1–1.7 | 0.256 |
| Prematurity | |||
| No | 1.0 | – | |
| Yes | 0.3 | 0.1–1.2 | 0.091 |
| Vaccine adverse events | |||
| No | 1.0 | – | |
| Yes | 0.3 | 0.1–1.1 | 0.077 |
| Other vaccines on the same day | |||
| No | 1.0 | – | |
| Yes | 3.3 | 1.0–10.7 | 0.050 |
Forty-two individuals were excluded due to missing data. Pseudo-R2=0.13, p=0.011.
This study identified 93.6% positivity for anti-HAV after a single-dose HAV vaccination, as adopted by the National Immunization Program of Brazil. This rate is comparable to those found in previous studies that used a single-dose.7,8,11,12 Although no assays have been performed to assess cellular immune response in the present study, other authors have shown that the CD4+ T cell, CD8+ T cell, and B memory cell responses against HAV are associated with the development of antibodies.16,17 Therefore, it seems that the strategy adopted in Brazil is achieving its initial targets.
The use of finger pricking for blood samples was a practical solution, well accepted by the community, and avoided loss of participants. EIA performed on samples obtained by DBS have proved useful for the detection of anti-HAV antibodies after both natural infection and vaccination. Sensitivity and specificity ranging from 62.5% to 89.6% and 97.5% to 100%, respectively, compared to the standard method of EIA using serum.16,18,19 Since the detection of anti-HAV using DBS is less sensitive than detection using venous blood serum, we got a second blood sample from venipuncture to increase sensitivity.19 Anyway, the DBS technique achieved good levels of detection and over 82% of participants did not require another blood sample collection.
Ten weakly positive samples were retested using serum. Reproducibility was 90%. In one case, it was not possible to confirm positivity for anti-HAV antibodies. Of note, the two samples taken were very close to the cut off, despite more than a year separating the two blood collections. Consequently, non-confirmation of the second test may be due to a small decrease of antibody titers over time.
In this study, it was not possible to retest all 47 individuals who had tested negative with the DBS technique. However, retesting 34 of these children showed that 18 (53%) of them were anti-HIV positive. For ethical reasons, children with two negative tests received a second dose of the vaccine. This is not a practice to be adopted in primary care unless further studies show that single dose vaccination is not as effective as two doses. It is anticipated that some individuals will not seroconvert after one dose of vaccine.2 This does not indicate a need for retesting the entire population or a flaw in the strategy adopted. Routine retesting of all vaccinated individuals would make the program expensive and would be of little practical advantage. Even those who have not seroconverted may be protected by herd immunity if the strategy is successful.
The analysis of the demographic and epidemiological aspects did not identify any determinant factor for anti-HAV seropositivity, except for the administration of more than one vaccine on the same day. However, the statistical significance was borderline (p=0.05), and the logistic model had little explanatory power (pseudo-R2=0.13). The small number (only 16) of non-seroconverted individuals may have contributed to this. As this association does not seem very plausible, it may have occurred randomly. A robust review involving more than 50,000 subjects showed no interference in the HAV vaccine immunogenicity in those who received other vaccines simultaneously.20
A limitation of the study was that the participants were not randomly included. Therefore, the sample was increased by twofold to reduce chances of bias and increase the study power. A further limitation is that the anti-HAV status was unknown before vaccination. Therefore, it is not possible to determine whether antibodies in anti-HAV positive individuals were induced by the vaccine or natural infection. In a randomized study performed over the last decade and including major cities in the Central region of Brazil, the authors reported an anti-HAV prevalence of 32% in children aged between 5 and 9 years.21 The prevalence among children aged between 1 and 3 years is likely to be considerably lower. Therefore, it is plausible to postulate that the vast majority of anti-HAV positive subjects developed these antibodies as a consequence of vaccination.
Finally, this survey showed that following the introduction of HAV vaccination in a small town in Brazil, more than 90% of children developed antibodies. This is the first evidence suggesting that the strategy adopted in Brazil using a single-dose of the vaccine may be sufficient for mass immunization. Nonetheless, it is too early to assess whether the immune response elicited will be persistent and whether the strategy will lead to decrease the endemic levels of illness in the country. Further studies are required to evaluate the single-dose strategy effectiveness.
Conflicts of interestThe authors declare no conflicts of interest.