The present study was aimed to identify the underlying mechanisms of improper renal function in Leishmania donovani infection that causes VL. Mice (BALB/c) were infected with L. donovani and different parameters for proteinuria were assessed. The levels of superoxide anion (O2−), hydrogen peroxide (H2O2), lipid peroxidation (MDA), inflammatory cytokines, and toll-like receptor (TLR) 2 and 4 expression were found significantly elevated at 60th day in these animals and declined at 90th day post infection. However, TGF-β and caspase 3 activities were higher at 90th day in comparison to 60th day post infection. These findings suggested that exacerbated inflammatory conditions correlate with abnormal renal functions in L. donovani infection, which is further augmented by activated TLRs expressions by circulating leishmanial antigens. Further, the increased levels of TGF-β and caspase 3 at 90th day suggested TGF-β mediated apoptotic cell death of renal and other cells during later stages of disease that may eventually result in release of host and parasitic factors in urine during visceral leishmaniasis.
The global annual burden of all forms of leishmaniasis is approximately 12 million per year and approximately 350 million people are at risk; however, exact statistical data are lacking.1 The disease is characterized by three clinical forms, i.e. visceral (VL), cutaneous (CL) and mucocutaneous (MCL) leishmaniasis. Out of these the visceral disease is almost always fatal, if left untreated. An estimated annual global burden of VL is about 0.2–0.4 and CL is approximately 0.7–1.2 million.1,2 Out of the total VL cases globally, about 90% occurs in India, Nepal, Bangladesh, Sudan, and Brazil.
Along with poor establishment of immune response, host kidneys are also affected in leishmaniasis. In chronic leishmanial infection the renal abnormalities are clinically characterized by impaired renal function, i.e. proteinuria and hematuria along with morphological changes such as crescent formation.3 However, the exact cause of mechanisms of renal damage and abnormal renal function is still unknown in visceral leishmaniasis. Oxidative stress is major determinant of various renal diseases and also contributes to the manifestation of glomerulonephritis. Immune response in renal cells can also be augmented by toll-like receptors (TLRs) in response to circulating antigens, which activate production of various inflammatory cytokines. TLRs have also been shown to be involved in the abnormal renal function in several diseases like tuberculosis, salmonellosis, and rheumatoid arthritis, but their role is largely unknown in visceral leishmaniasis.4 TLRs signaling occur via MyD88 dependent or independent pathways that lead to the activation of either p38 or ERK1/2 related mitogen activated protein kinases (MAPK) and subsequent production of either inflammatory cytokines such as TNF-α, IFN-γ, and IL-12 or anti-inflammatory cytokines such as IL-4, IL-10.5 Many studies also suggest the importance of TLRs signaling in the onset of leishmanial pathogenesis, resistance and susceptibility; however, limited data exist on their expression in the kidney and their role in renal damage in L. donovani infection.6
Proteinuria is also associated with apoptotic death of renal cells.7 TNF-α induced TGF-β mediated apoptotic cascade has been found as one of the major pathway that leads to renal cells death.7 TGF-β is also shown to be involved in pathogenesis of renal diseases via stimulating the synthesis of extracellular matrix components and decreasing collagenase production but its role is yet not examined in L. donovani induced renal abnormality.8 In this study, we measured the levels of oxidative stress markers, TNF-α, IFN-γ, IL-12, TGF-β, and expression levels of TLR 2, 4, caspase 3 in renal tissues of L. donovani infected mice with the symptoms of proteinuria.
Ten-week-old female BALB/c mice were used in this study. This study was conducted following principles of laboratory animal care guidelines and approved by institutional animal ethical committee. The animals were kept in polypropylene cages with chopped wheat straw as the bedding material and temperature around 20–30°C. They were fed with a standard chow and water ad libidium. L. donovani promastigotes were cultured to obtain metacyclic forms in Schneider's insect media (pH – 5.5) supplemented with 20% fetal calf serum for 24h in a BOD incubator as previously described.9 Animals (8, in each group) were infected with metacyclic promastigotes (2×107 per ml), intravenously for the development of VL pathogenesis. After three weeks, confirmation of leishmanial pathogenesis was done in infected animals by rk39 strip test and demonstration of parasites in splenic aspirates by Giemsa staining.
For histopathological observations, the kidney sections were hematoxylin–eosin stained and observed under bright field microscope. For estimations of urinary proteins, the urine samples were collected using clear plastic wrap method described by Kurien and Scofield for further analysis.10 As soon as the animal urinated, the animal was removed and the urine was collected. Protein concentrations were determined by a modified Bradford method, adapted to a microtiter plate assay as previously described.11 The 24h urinary protein excretion was calculated from the 24h volume and urinary protein concentration. Serum and urine creatinine concentrations were measured by the alkaline picric acid method using an autoanalyzer.
All other biochemicals were estimated in kidney tissue lysates in all experimental animals comprising eight mice in each group (control, 60th and 90th day post infection). All parameters were measured at 60th and 90th day post infection. The kidneys of mice without infection were used as control. To prepare tissue lysate, kidney tissue (∼50mg) were homogenized in Tris–HCl buffer (pH – 8.0) containing leupeptin (10μg/ml), aprotinin (10μg/ml), Triton X-100 (1%), PMSF (1mM), EGTA (1mM), NaF (5mM), and sodium orthovanadate (10mM). The homogenized samples were centrifuged at 10,000rpm for 10min at 4°C. The protein content in supernatant was measured by Bradford method11 and stored at −80°C for measurements of various oxidants, cytokines, and TLRs. The superoxide anion content was estimated by the method described elsewhere12 in 100μl of kidney lysate supernatant and expressed as nmoles of O2− liberated per mg of protein. The production of hydrogen peroxide (in 100μl lysate) was measured fluoremetrically according to the method described elsewhere13 represented in nmole/mg of protein. LPO was measured according to standard protocol as described previously in 500μl of lysate14 and lipid peroxidation (LPO) was expressed as nmoles of MDA/mg of protein. The levels of TNF-α, IL-12, IFN-γ, and TGF-β were estimated by ELISA MAX™ standard set enzyme-linked immunosorbent assay kit as per manufacturer's instructions (Biolegend, USA). The renal cytokine levels were normalized to protein concentration of lysate and expressed in terms of pg of cytokines/mg of protein. The caspase 3 activity in renal tissue was measured by caspase 3 fluorimetric assay kit (Sigma Chemicals, USA) as per manufacturer's instruction. The caspase 3 activity was and expressed in picomoles per minute per μg of protein.
TLR 2, 4, and caspase 3 mRNAs were quantified by real time PCR on ABI700Fast cycler. Total RNA from kidney tissues (∼50mg) was extracted by RNeasy Mini kit (Quaigen Cat. No-74104) following manufacturer's instructions. RNA pellets were washed thrice with 70% DEPC ethanol and digested with RNAse free DNase (Fermantas, Germany). For cDNA preparation, 1μg total RNA (kept equal for each amplification) was subjected to reverse transcription using 20U M-MLV reverse transcriptase Fermantas, Germany), 1× RT buffer, 20mM dNTPs (New England Biolabs, USA), 20U RNasin (Fermentas, Germany), 0.1M DTT with DEPC treated water, and 100ng of random hexamers (Fermentas, Germany). The expression levels were quantified on ABI7500Fast system as per manufacturer instructions (Applied Biosystem) using mice mRNA specific forward (TLR 2: 5′-TCTGGCTCAAATCCTGGTTG-3′, TLR 4: 5′-TGGGTGAGAAATGAGCTGGT-3′, caspase 3: 5-GAGCAGCTTTGTGTGTGTGA-3′) and reverse (TLR 2: 5′-GCACCTACGAGCAAGATCAA-3′, TLR 4: 5′ACCACAATAACCTTCCGGCT-3′, caspase 3: 5′-TTCGGCTTTCCAGTCAGACT-3′) primers (5pmol/μl). The β-actin gene (F: 5′-AACCGCGAGAAGATGACCCAGATCATGTTT-3, R: 5′-AGCAGCCGTGGCCATCTCTTGCTCGAAGTC-3′) was used as housekeeping control. Briefly, 20μl of real time mix contained 10μl of Power SYBER green master mix (Applied Biosystem), 1μl cDNA, 1.5μl each, forward and reverse primers, 6μl MilliQ water. PCR conditions were set with an initial incubation of 50°C for 2min, followed by denaturation at 95°C for 10min, and 40 cycles at 95°C for 15s, 60°C for 1min, and 72°C for 40s. The abundance of mRNA was normalized to geometric average of endogenous control (β-actin) for ΔCt, and results were expressed in comparison to non-infected animals.
The data were analyzed by one-way analysis of variance (ANOVA) using Students Newmann–Keuls (SNK) test and mRNA fold change were analyzed by Student's two-tailed t test using GraphPad Prism 5.0 software. All the experiments were performed in triplicate, and data are represented as mean±standard deviation.
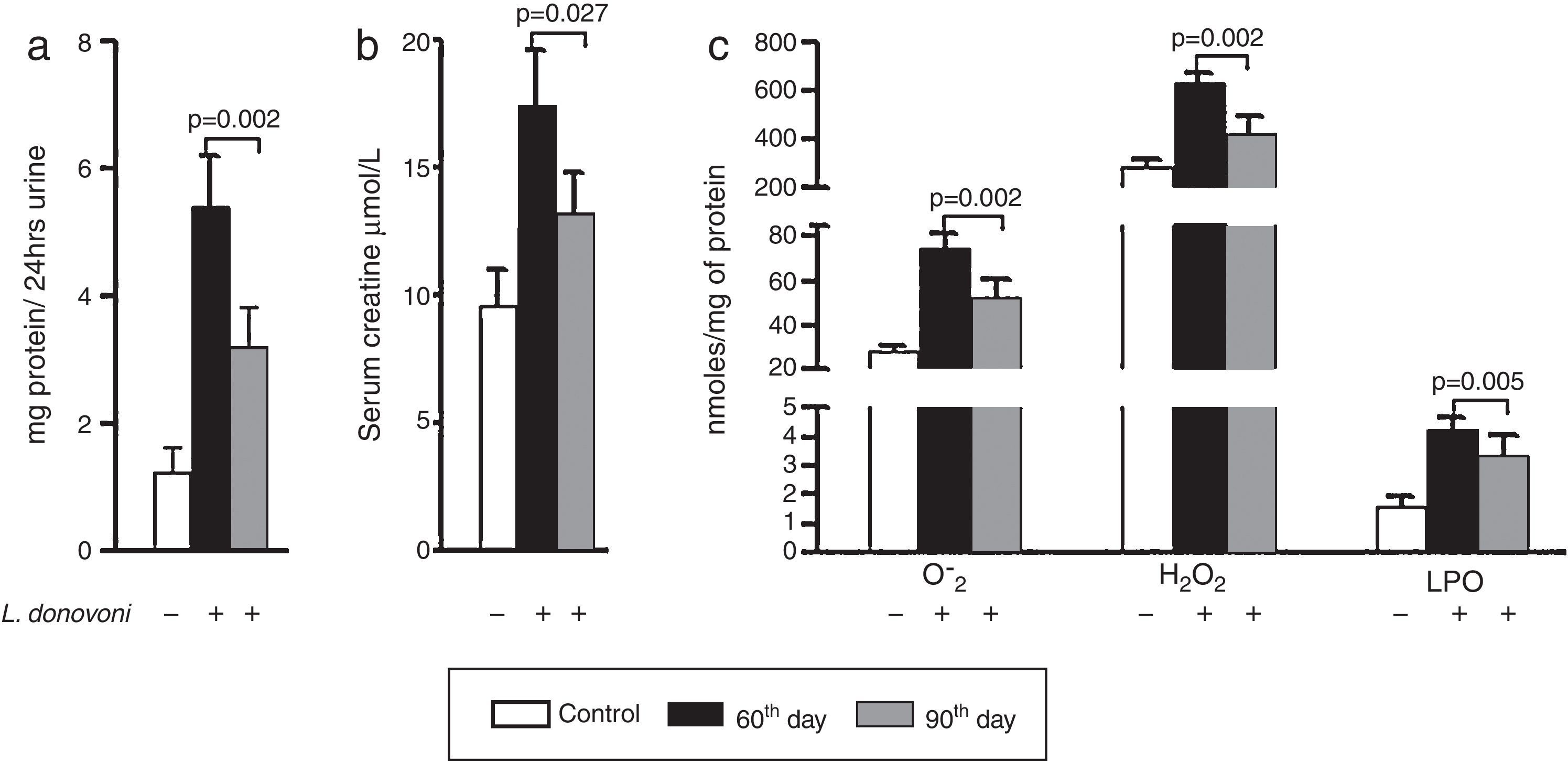
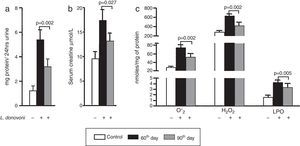
The levels of protein in urine and serum creatinine of all three experimental groups are depicted in Fig. 1A and B, respectively. At 90th day post infection proteinuria (p=0.002) and serum creatinine (p=0.027) were significantly decreased as compared to 60th day post infection. The levels of oxidative stress markers, i.e. O2− (p=0.002), H2O2 (p=0.002), and MDA (p=0.005) were significantly higher at 60th day post infection (Fig. 1C). These results were also correlated with histopathological observation of kidney in infected animals. At day 60 post infection, we observed increased hypercellularity and reduced Bowman's space (Supplementary Figure 1). However, post 90 days the glomerular cellularity was found reduced.
(A, B) Urine protein (p=0.002) and serum creatinine (p=0.027) levels were found multifold increased on 60th day while at 90th day there was sharp decline in their levels. (C) The generation of super oxide anions (p=0.002), H2O2 (p=0.002), and lipid peroxidation levels (p=0.005) were also found significantly increased at 60th day while at 90th day there was sharp decline in their levels.
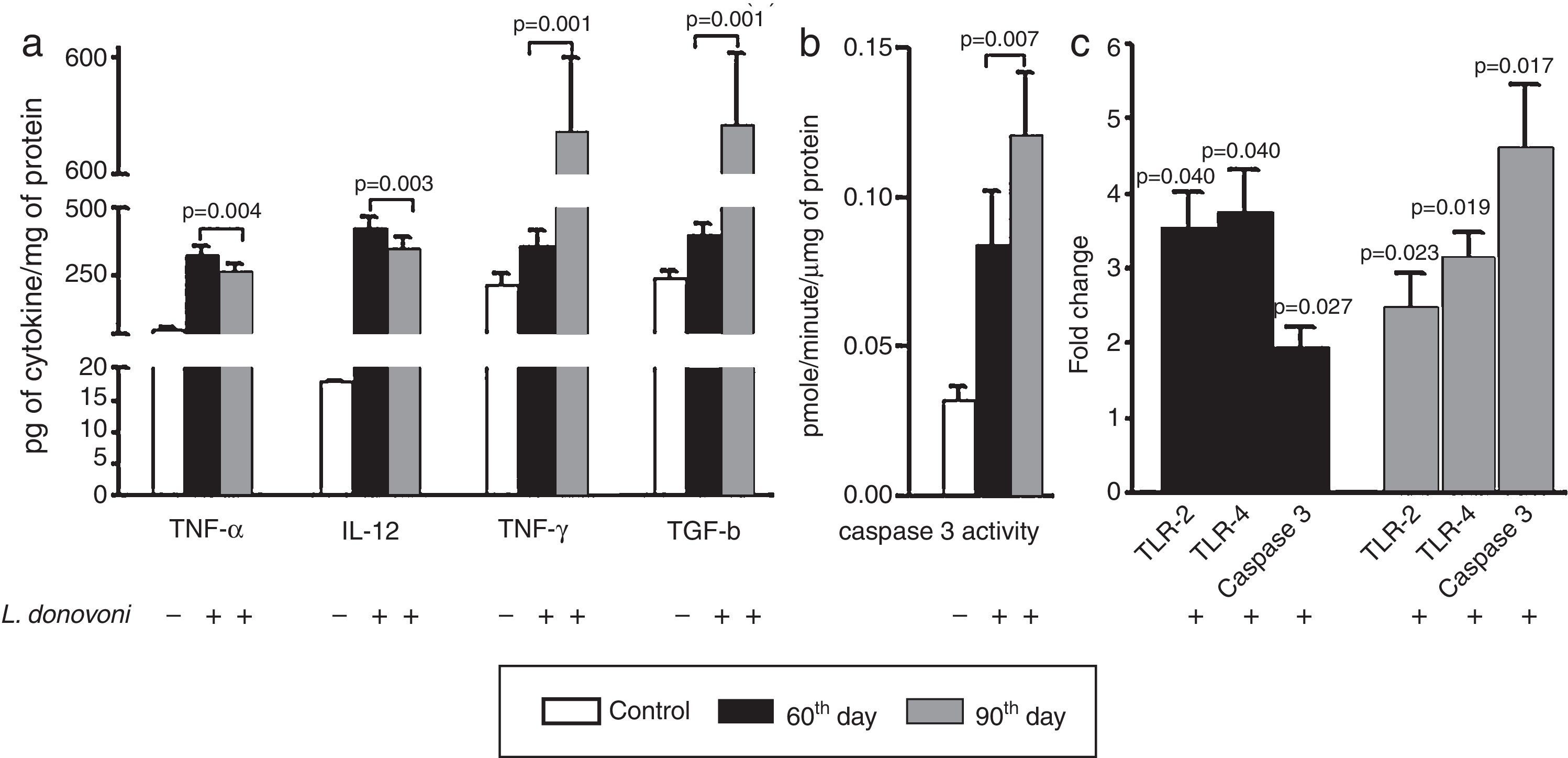
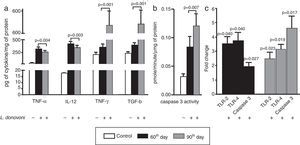
The cytokine levels are depicted in Fig. 2A. At 60th day, the levels of TNF-α (p=0.004), IL-12 (p=0.003), and IFN-γ (p=0.001) were also higher as compared to 90th day. However, a TGF-β level was found significantly (p=0.001) higher at 90th day as compared to 60th day post infection. The caspase 3 activity was found significantly (p<0.001) more elevated in infected animal as compared to controls, and was highly (p=0.007) elevated at 90th day among parasite infected animals (Fig. 2B).
(A) The levels of TNF-α (p=0.004) and IL-12 (p=0.003) were found increased in infected animals at 60th day post infection as compared to 90th day post infection. The levels of IFN-γ (p=0.001) and TGF-β (p=0.001) were found increased in infected cells at 60th day as compared to 90th post-infection. (B) The caspase 3 activity was significant (p<0.001) increased in infected animal versus control. At 90th day, caspase 3 activity was significantly (p=0.007) higher than 60th day. (C) The levels of TLR 2 and 4, and caspase 3 were measured at 60th and 90th day post infection. At 60th day post infection, there was up regulation of TLR 2, 4, and caspase 3 with fold change 3.535±0.4906 (p=0.0407), 3.765±0.5650 (p=0.0466), and 1.956±0.2706 (p=0.0257). Similarly on 90th day there was up regulation of all these, TLR 2, 4 and caspase 3 in comparison to non-infected controls with fold change 2.507±0.4523 (p=0.0235), 3.165±0.3146 (p=0.0194), and 4.622±0.8303 (p=0.0171), respectively.
The levels of TLR 2, 4, and caspase 3 were measured at 60th and 90th day post infection and results were compared to non-infected animals (Fig. 2C). At both, 60th and 90th day post infection, there was up regulation of TLR 2 (day 60; p=0.0407, day 90; p=0.0235), TLR 4 (day 60; p=0.0466, day 90; p=0.0194), and caspase 3 (day 60; p=0.0257, day 90; p=0.0171) in comparison to non-infected controls. On comparison between 60th and 90th post infection, the TLRs mRNA levels were similar; however, caspase 3 expression level was significantly higher at 90th day post infection.
The factors involved in VL associated with proteinuria and abnormal renal functions are still unexplored. The findings of the present study suggested that leishmanial circulatory antigens may induce renal inflammatory conditions via TLRs activation that allows more proteins to cross the glomerular barrier. This study revealed that exacerbated inflammatory conditions in renal tissues along with TGF-β mediated apoptosis play significant role in development of abnormal renal function in L. donovani infection.
The purpose of host oxidative assault is to eliminate parasitic intrusion, but their persistent production may also lead to organ or tissue damage. Reactive oxygen species (O2−, H2O2, etc.) are potentially damaging moieties that bring functional and structural modification of proteins, lipid peroxidation, which further alters the orderly architecture of cells. These radicals also help in activation of inflammatory response by regulating tyrosine and mitogen activated protein kinase (MAPK) and activation of transcriptional factors like NF-κB, AP-1, which lead to production of both inflammatory and anti-inflammatory cytokines.15Leishmania and leishmanial antigens are known to activate TLRs and subsequent production of inflammatory cytokines via activation of p38 MAPK kinase and anti-inflammatory cytokines via activation of ERK1/2.6,16,17 In this study, we observed increased production of inflammatory cytokines, which suggested the activation of p38 MAP kinase by persistent leishmanial proteins in renal tissues that further augment of TNF-α, IFN-γ, and IL-12 production.17 The exacerbated production of free radicals and inflammatory cytokines can also increase vascular permeability as well as compression of peritubular capillaries, which further propel initial injury and death of renal cells during Leishmania infection.
Apoptosis also plays a key role in determining the outcome of glomerulonephritis, which is generally characterized by increased production of TGF-β, a known apoptotic inducer, in renal tissues that also act as anti-inflammatory cytokines. It binds to its membranous receptors on the renal cell surface that lead to recruitment and activation of smad, smad 2, and smad 3 signaling proteins. After activation, these apoptotic smad proteins bind to cytosolic smad 4, which eventually forms a complex and activates target genes responsible for apoptosis.18 This study suggested the possible role of TGF-β mediated apoptotic death of renal cells, which eventually results in cellular disorganization as well as renal tissue damage and subsequent release of parasitic and host factors/proteins in patients urine.19 TGF-β also acts as an endogenous defender against IL-1β, TNF-α, and MCP-1 production.20 We also observed strong negative correlation between the levels of TGF-β and inflammatory cytokines. In experimental leishmaniasis, the leishmanial infections are generally self-healing, and it is quite likely that TGF-β mediated suppression of inflammatory response play important role in these animals. However, more studies are required to understand the role of TGF-β mediated apoptosis and its correlation between productions of inflammatory cytokines in L. donovani infection. Therefore, an attempt should be made for early and effective control of Leishmania infection to avoid renal complications and mortality in visceral leishmaniasis.
Conflicts of interestThe authors declare no conflicts of interest.
The financial support received from Department of Science and Technology, New Delhi (SB/SO/HS/0091/2013) is greatly acknowledged. The author VK is thankful to DST (YSS/2015/000687) and ICMR (F/839/2011/ECD-II), New Delhi, India, respectively for their research fellowship.