Mayaro virus is an alphavirus from the Togaviridae family and is transmitted mainly by Hemagogus mosquitoes. This virus circulates in high-density tropical forests or rural areas of Central and South America causing a disease characterized by high-grade fever, maculopapular skin rash and marked arthralgia that, in some patients, can persist for long periods after infection and may be misinterpreted as chikungunya. Although only a few outbreaks involving this virus have been reported, in the last years the number of Mayaro virus infections has increased in the central and northern regions of Brazil. In this review, we describe the reported prevalence of this infection over the years and discuss the circumstances that can contribute to the establishment of an urban mayaro virus epidemic in Brazil and the problems encountered with the specific diagnosis, especially the antigenic cross-reactivity of this pathogen with other viruses of the same family.
Mayaro virus (MAYV; genus Alphavirus, family Togaviridae) is an arbovirus transmitted primarily by the bite of female mosquitoes of the genus Hemagogus, usually from an infected non-human primate (monkeys) to a susceptible human. These tree-dwelling mosquitoes are also the vectors involved in the sylvatic cycle of yellow fever virus, and although MAYV maintenance efficiency in primary reservoirs is not known, it has been detected in nature in several vertebrate hosts such as non-human primates, rodents, birds, sloths, and other small mammals.1
As for yellow fever virus, MAYV can infect humans who inhabit the rural areas or enter these forested areas either to work or to take advantage of the environmental attractions, leading to a febrile condition characterized by fever, cutaneous rash, and joint pain. In recent years, there has been an increased number of cases of mayaro fever in several Brazilian states and with the increased circulation of chikungunya virus, there is always a question as to whether a febrile exanthematous disease with severe joint involvement is due to chikungunya or MAYV. In this review, based on epidemiologic and laboratory data, we discuss the variables involved in the possible establishment of outbreaks caused by MAYV.
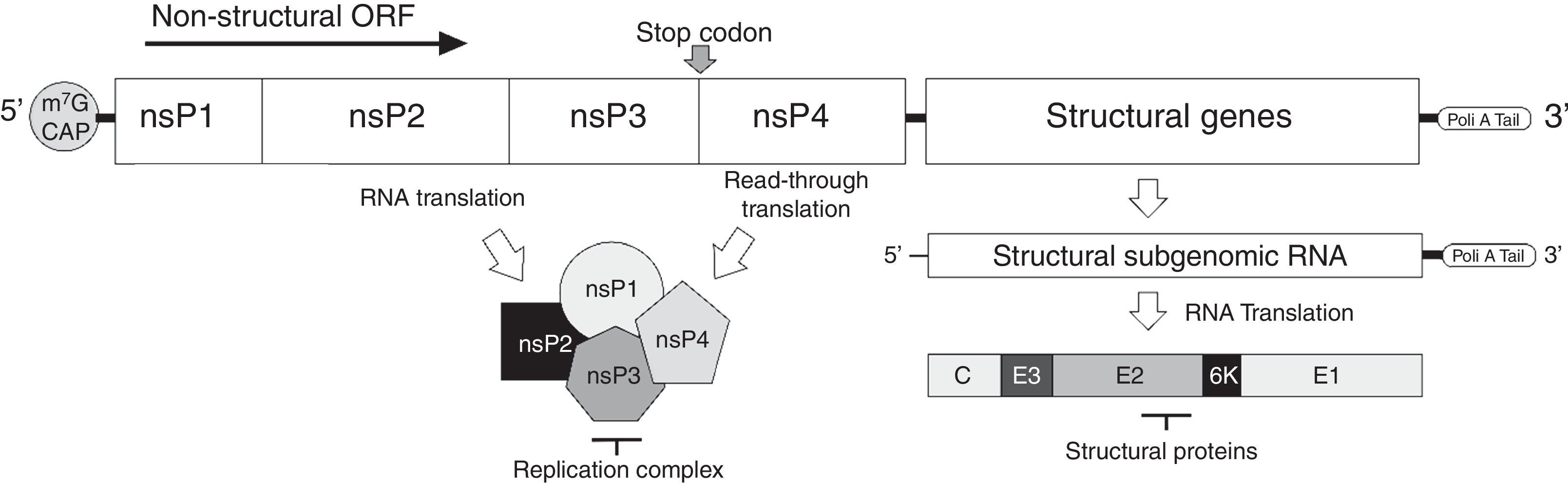
The virusMAYV genome is composed of a single-stranded positive-sense RNA of approximately 12kb in size, flanked by a 5′-7-methylguanylate (m7G) cap and a 3′-poly-A tail. It encodes four nonstructural proteins (nsP1–4) and five structural proteins (C-E3-E2-6k-E1).2 Similar to other alphaviruses, MAYV RNA has a stop codon at the end of nsP3 gene, and at a low frequency (5–20%), a read-through translation can occur, resulting in nsP1–4 product (Fig. 1). Structural proteins are produced after translation of a subgenomic RNA transcript, synthesized from the negative-sense RNA used for replication, a common feature of the Togaviridae family3 (Fig. 1). Following the expression of structural proteins, the virus RNA is complexed to the capsid protein and the envelope proteins, processed during their traffic through the Golgi apparatus, are inserted into the plasma membrane from where the virus acquires its envelope, releasing infectious particles able to infect susceptible cells.
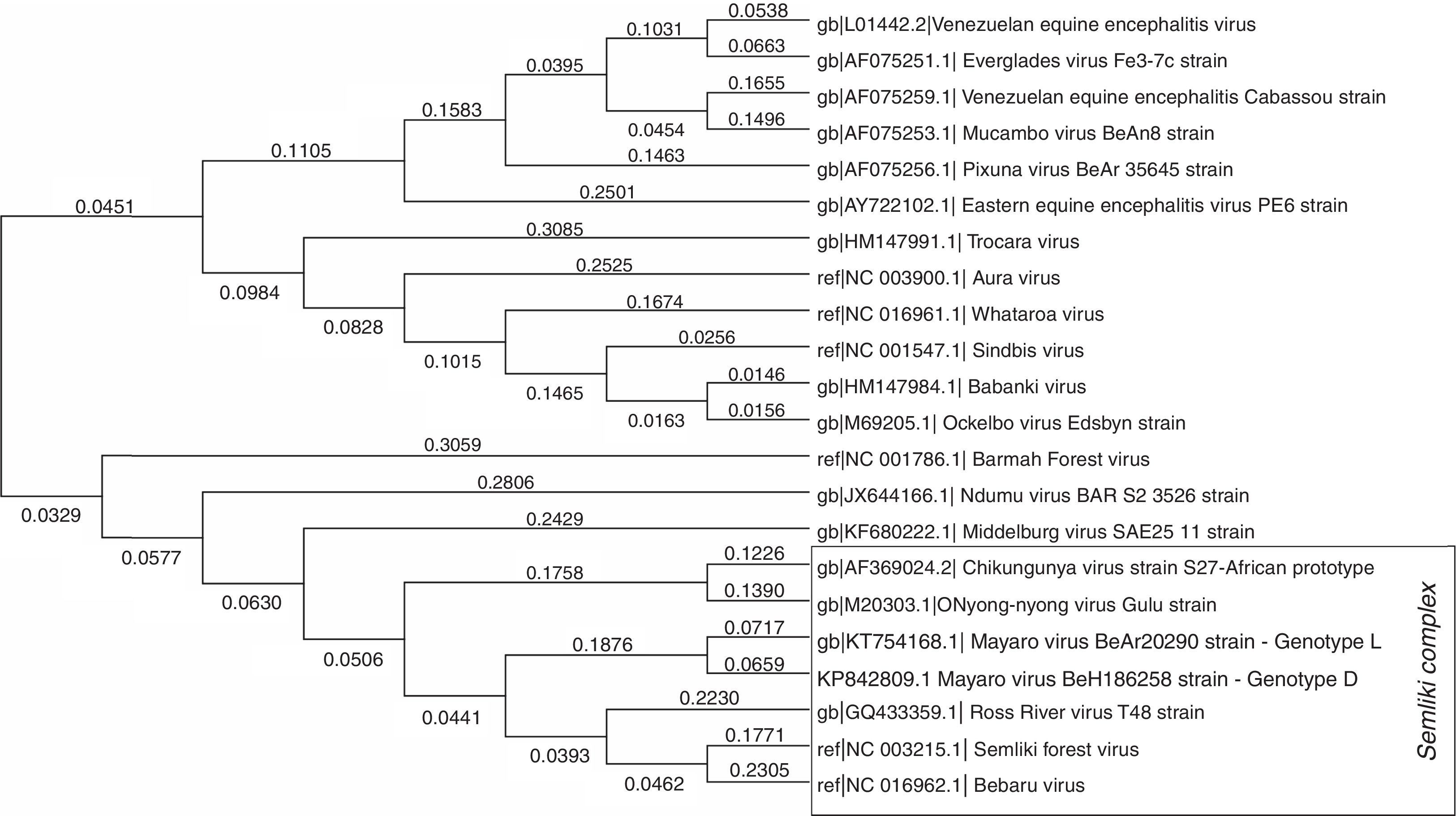
Phylogenetically, MAYV is considered a monophyletic group, divided in two similar genotypes–genotype D, which includes most of the isolates, from 1954 to 2003, and genotype L, limited to the Brazilian isolates.4 MAYV is part of the Semliki Complex, a serological group inside Alphavirus genus that shares some common antigenic sites, which can generate cross-reactivity with polyclonal immune sera among the species, when analyzed by conventional serological tests, such as Hemagglutinin Inhibition (HI) and Complement Fixation (CF). The Semliki Complex is composed by eight viruses (Bebaru, Chikungunya, Mayaro, Getah, Semliki Forest, Ross River, O’nyong-nyong, and Una viruses) of veterinary and medical importance, usually causing human disease characterized by fever, arthritis and skin rash5 (Fig. 2 – the strain of MAYV used to assemble this phylogeny was BeAr202906). As mentioned before, the fact that these viruses belong to the same serological complex, the correct diagnosis of these infections can be difficult to achieve, usually only carried out in well-equipped research laboratories. Specifically, of importance to Brazil, chikungunya and mayaro fever can exhibit the same clinical manifestations and both viruses are co-circulating in the country.
Molecular phylogenetic analysis of some clinically important alphaviruses using Maximum Likelihood method. A representative strain of each species, with a complete genome deposited at NCBI website, was used to construct the phylogenetic tree. A total of 9946 positions were analyzed in the final dataset. The numbers above branches indicate the evolutionary distance (branch length). Evolutionary analyses were conducted using MEGA7 software. The strains of mayaro virus used to assemble this phylogeny were BeAr20290(5) for genotype L and BeH186258 for genotype D.
Little is known about the pathophysiology of mayaro fever, but once the infection has established the virus goes through an intrinsic incubation period, which can range from 3 to 11 days, when a high-rate replication takes place followed by a short viremia lasting while the symptoms are present (usually 5–7 days). The symptoms of MAYV infection are often an exanthematous rash, fever, myalgia, retro-orbital pain, headache, diarrhea, and arthralgia, the latter may persist for months or even years.7 There are several reports on the literature of persistent or recurrent arthralgia resulting from MAYV infection,8–10 which could be a result of an inefficient neutralizing antibody response against this virus associated with the continuous expression of some pro-inflammatory immune cytokines.11 However, there is a need for more studies evaluating the broad spectrum of the clinical manifestations of the acute phase of the disease, including unusual presentations, if present, as well as the possible chronic clinical manifestations, and also, the viral and immunological parameters involved in the causation of each form of this disease.
As for most arboviral infections, the diagnosis of alphavirus infection, such as Mayaro fever, is more efficient if performed during the acute phase of the disease, either by virus isolation or RT-PCR to detect viral RNA. At a convalescent phase of infection, antibody cross-reactivity has been shown to occur when using traditional serological tests, such as ELISA. High cross-reactivity between mayaro and chikungunya has been described,12,13 and either side-by-side tests aiming at the comparison of serum reactivity to each virus or neutralization tests should be performed to correctly establish the specificity of an antibody reaction, and provide a correct diagnosis of the disease.
EpidemiologyThe epidemiologic history of MAYV infection begins in 1954 with its first report in Trinidad and Tobago, when the virus was isolated from blood samples of five rural workers that presented with a febrile disease. The virus received its name after Mayaro County, a southeastern region of Trinidad, where the cases were reported.14 Since then, the virus has been reported in some countries in Central and South America, usually in places with tropical forests, such as French Guiana, Bolivia, Peru, Suriname, Costa Rica, Guatemala, Venezuela, Mexico, Ecuador, Guyana, Panama, and Brazil. Imported cases are not very frequent, but some have been reported: North Americans visiting Peru15 and Bolivia10; French citizens returning from French Guiana16 and Brazil17; a German woman returning from Bolivia18; a Swiss tourist visiting Peru19; a Dutch couple returning from Suriname,20 and some interstate imported cases in Brazil.21,22 The virus is considered endemic in some places of Brazil, such as the northern, central, and western regions of the country. The first case in Brazil was reported in 195523 next to Guama River, Pará state, and the first epidemic in Brazil occurred in a village next to that river. A little more than two decades after that, in 1978 in Belterra, Pará, an outbreak was described with 55 confirmed cases (43 with virus isolation and 12 with serological tests) from a total of 72 individuals with acute illness (fever and arthralgia were present in most of them).24
Other two outbreaks have been reported by Vasconcelos et al.25 in Conceição do Araguaia in 1981 and Benevides in 1991, both cities in Pará state, northern Brazil, and in Peixe, Tocantins state in 1991. In the beginning of 2008, an outbreak broke out in a settlement in Santa Barbara and surroundings, Pará state. From a total of 105 individuals that reported a febrile condition in the past 30 days, 36 had IgM antibodies against MAYV.26 It was reported the presence of IgM antibodies against MAYV in 33 patients, and viral genome was detected from one of them in Manaus, during an acute febrile outbreak in 2007–2008.27 Also, during a dengue virus outbreak in Mato Grosso, Central-West region of Brazil, 15 out of 604 patients were positive for mayaro RNA detection during an acute febrile illness.28
More recently, the state of Goiás experienced another outbreak of the disease, and about 183 cases have been notified. From December 2014 until January 2016, a total of 343 suspected cases were notified as a result of MAYV infection in Brazil, in which more than 50% were from Goiás State.29 Some of those cases were initially reported as chikungunya infection, since both viruses are closely related to each other and there is antibody cross-reactivity in the available diagnostic tests. As an evidence for this cross-reactivity, a large number of mayaro fever cases that occurred in this outbreak were positive for both viruses by serologic tests.
The increasing incidence of mayaro fever in regions of the country other than the northern region, where the disease is endemic, is a growing concern because this can indicate that the virus is spreading to other parts of the country, and future epidemics may occur in Brazil, in areas where health care workers are not familiar with mayaro fever clinical presentation.
Laboratory studiesEvidence that mosquitoes from genus Aedes could transmit MAYV is another circumstance that may contribute to the establishment of mayaro fever outbreaks in Brazil, since most of the country is infested by both Aedes aegypti and Aedes albopictus. Laboratory experiments have shown that, indeed, MAYV can infect Ae. albopictus, determining a productive viral replication not only in vitro, but in vivo, and then, transmit the virus.30,31 Similar to Ae. albopictus, Ae. aegypti showed to be an effective vector to transmit MAYV. After laboratory infection of mosquitoes, the virus could be found in the salivary glands and could be successfully transmitted to mice, at a high rate of infection.32 In addition to laboratory infection of Aedes mosquitoes by MAYV, Serra et al.33 found MAYV-infected Ae. aegypti and Culex quinquefasciatus in their natural habitats, in Cuiabá, Mato Grosso, Brazil. All these findings underscore the possibility of MAYV infection of Aedes sp. mosquitoes and the transmission cycle of this virus may shift from sylvatic to urban, using humans as amplifying hosts, and then establishing new outbreaks in Brazil, since the majority of the Brazilian population is susceptible to MAYV infection.
Prevention and control of MAYV infectionsThere is no antiviral against MAYV and only symptoms are treated, similar to treatment available for chikungunya virus infection, with the initial prescription of nonsteroidal anti-inflammatory drugs and analgesics for pain and fever relief, and sometimes corticosteroids, although its efficacy has not been fully proven.34 Not much data is available on MAYV treatment, but drugs and therapies are administrated similar to other alphavirus infections that have a clinical course comparable to mayaro infection, such as chikungunya and Ross River viruses.
No commercial vaccines are available, but two candidates were tested in pre-clinical experiments. The first attempt generated inactivated particles, using different concentrations of formalin and showed to be immunogenic when administrated in mouse, with some efficacy.35 Another candidate was a live attenuated vaccine, that could replicate in vertebrate cells, but not in mosquitoes, with mutations in sub-genomic promoter and translational control of an internal ribosomal site (IRES) from encephalomyocarditis virus (EMCV), which demonstrated a great production of neutralizing antibodies and a high rate of survival after challenge, using immunocompetent mice.36
ConclusionMAYV is the cause of an emergent infection in Central and South America, and similar to what happened to zika and chikungunya viruses, it has the potential to establish an epidemic scenario in Brazil. Although there is no evidence of the transmission efficiency of MAYV in an urban cycle, the susceptibility of the population to this infection, in addition to the possibility of transmission of this pathogen by Aedes mosquitoes, widely distributed in Brazil, can increase the number of infected individuals. Due to its antigenic similarity with chikungunya virus, it is clear the urgency for the development of specific and accurate diagnostic methods for a better diagnosis of MAYV infections. In the possession of accurate diagnostic tests, it will be possible to distinguish the infections caused by MAYV from other arboviral infections and define the real importance of MAYV to the Brazilian public health and to define the complete picture of mayaro fever clinical manifestations.
Since 2014, a growing epidemic of chikungunya virus infection is occurring in Brazil. Data from the Brazilian Ministry of Health show almost a 10-fold increase of suspected cases occurred in Brazil, from the year of 2015 to 2016 (38,499 to 265,554 cases, respectively),37 with most of the cases being reported in the northern and northeastern regions of the country, where MAYV is endemic. Most of these cases were diagnosed by a clinical–epidemiological criterion, since there are no reliable and specific serological tests to confirm the infection. Considering that no difference can be found in symptoms from both infections, the number of cases of mayaro fever could be underestimated and many of the chikungunya cases were really MAYV infections.
In terms of controlling possible mayaro fever outbreaks, since there is no vaccine to suppress the spread of this disease, the only current approach is vector control. However, this approach has proved so far inefficient, in the view of the increasing number of new arboviral infections (dengue, zika, chikungunya, and even mayaro) in the country in the last years. Finally, in order to lower the importance of these diseases to public health, it is of paramount importance the development of a vaccine or an antiviral treatment to alphavirus infections, due to the impact mayaro and chikungunya fever may have on the quality of life of people infected with these viruses.
Conflicts of interestThe authors declare no conflicts of interest.