The objective of this study was to determine risk factors associated with mortality in patients with nosocomial Escherichia coli bacteremia from January 2009 to January 2011. In a retrospective study the medical records of 88 patients over 18 years with nosocomial bacteremia caused by E. coli were analyzed. In univariate analysis several risk factors, including chronic renal failure, altered mental status, leukocytosis, and higher Charlson index of comorbidities were associated with mortality. In multivariate analysis only altered mental status remained independently associated with mortality. Mental confusion can be a risk factor for mortality in patients with E. coli bacteremia.
The main risk factors for bacteremia caused by Escherichia coli include admission at the intensive care unit, previous antibiotic use, invasive disposal (mainly urinary catheter) and comorbidities. The mortality of bacteremia caused by E. coli acquired in the hospital is greater than when acquired in the community acquired.1 Furthermore, mortality can be associated with source of bacteremia, which is lower when originated from the urinary tract.2 The objective of this report was to determine the predictive risk factors for mortality in E. coli bacteremia.
From January 2009 to January 2011 a retrospective cohort study was carried out at the Hospital Universitario Evangelico de Curitiba, a reference center for trauma, burn and renal transplant. This center is a 660-bed tertiary-care hospital in Curitiba, a city located in Southern Brazil with 60 intensive care beds. This study was approved by the Ethics Review Board (CONEP PR – 448689). Patients with nosocomial bacteremia caused by E. coli were evaluated. Exclusion criteria were age<18 years, previous bacteremia, bacteremia occurring before 72h of admission. Cultures were collected and processed using the BACT/Alert® (bioMérieux, Durham, USA). E. coli was identified using standard biochemical tests. Susceptibility testing was performed by the disk diffusion method according to the current CLSI guidelines.3
The following variables were evaluated for each patient: sex, age, previous hospital admission within 90 days, admission to the intensive care unit, length of hospitalization before bacteremia, central venous line, surgery during the current hospitalization, underlying conditions such as diabetes mellitus, chronic renal failure (with or without dialysis), heart failure, neoplasm, chronic obstructive pulmonary disease, HIV infection, cirrhosis, trauma and burn. Previous or concomitant infection by E. coli was evaluated. The susceptibility to quinolones and the presence of extended-spectrum beta-lactamases were included in the analysis. We also included Charlson comorbidity index score, previous use of antibiotics in the same admission, mental status through Glasgow Coma Scale (GCS) in the day of the bacteremia as clinical data. The following laboratory results were evaluated on the day of bacteremia: hemoglobin level, leukocyte and platelet counts, percentage of immature cells, creatinine, and total bilirubin. Thirty-day in hospital mortality was registered. Attributed mortality was not evaluated. Antibiotic treatment was classified as correct or incorrect, considering as correct if E. coli was susceptible to the antibiotic used during bacteremia. We evaluated separately the time from positive blood culture to start of the correct antibiotic (<24h, <48h, <72 and <5 days).
Continuous data were expressed as mean±standard deviation (SD) or median with ranges (when a considerable discrepancy was observed between mean media). Frequencies were expressed as percentages. Dichotomous variables were compared using Chi-square and Mann Whitney test was used for continuous variables. Significance level was set at 0.05. Variables with p<0.10 in univariate analysis were included in the multivariate analysis using binary logistic regression model. Odd ratios (OR) with 95% confidence intervals (95% CI) were calculated for each variable. Variables in which 95% CI did not include 1.0 were maintained in the final model. Kaplan–Meier survival estimates were calculated for risk factors found in the multivariate analysis and the difference was assessed using the log-rank test. Logistic regression was used to predict a score of mortality in patients with E. coli bacteremia according to the risk factors. All data were stored using the software Excel (Microsoft, New York, USA) and statistical analysis was performed using the software SPSS 16 (SPSS, Chicago, USA). Kaplan–Meier survival estimates were determined with GraphPad Prism 5.0 (GraphPad, La Jolla, USA).
Eighty-eight cases were enrolled in the study. The median days of hospitalization before bacteremia was 16 (range=3–36 days) with inter quarter percentiles (25% and 75%) between 4.2 and 20 days, respectively. The mean age was 59.7±17.3 (range=18–96) years and 52.3% were female. Nominal data are detailed in Table 1. Only seven cases (8.0%) of ESBL-producing E. coli were isolated. The total duration of hospitalization was 24.6±24.0 days. Most patients had anemia (mean hemoglobin 10.3±2.3g/L), leukocytosis (14.3±11.8×109/L) with increased percentage of immature cells (17.8±14.7%), renal failure (mean serum creatinine of 3.7±7.2 and media of 1.5, range 0.8–4.8) and hiperbilirrubinemia (mean total bilirrubin of 2.9±3.9 and media 1.4, range 0.8–2.8).
Clinical findings of nominal variables of 88 cases of Escherichia coli bacteremia – 2012.
| Data | n=88 | % |
|---|---|---|
| Gender | ||
| Male | 42 | 47.7 |
| Female | 46 | 52.3 |
| Previous admission within 90 days | 12 | 12.5 |
| Final mortality | 33 | 37.5 |
| ESBL-producing strains | 7 | 8.0 |
| Quinolone-resistant strains | 20 | 22.7 |
| Previous admission to ICU | 3 | 3.4 |
| Central venous catheter | 10 | 11.4 |
| Surgery | 8 | 9.1 |
| Diabetes mellitus | 23 | 26.1 |
| Chronic renal failure | 21 | 23.9 |
| Heart failure | 7 | 8.0 |
| Essential systemic hypertension | 32 | 36.4 |
| Cancer (solid/leukemia/lymphoma) | 21 | 23.9 |
| Charlson score | ||
| 0 | 40 | 45.5 |
| 1 | 3 | 3.4 |
| 2 | 35 | 39.8 |
| 3 | 4 | 4.5 |
| 4 | 6 | 6.8 |
| Trauma | 4 | 4.5 |
| HIV | 0 | 0.0 |
| Chronic obstructive pulmonary disease | 2 | 2.3 |
| Cirrhosis | 0 | 0.0 |
| Previous infection | 8 | 9.1 |
| E. coliin other sites | 11 | 12.5 |
| Abscess | 1 | 1.1 |
| Peritoneal fluid | 1 | 1.1 |
| Catheter tip | 7 | 8.0 |
| Urine | 2 | 2.3 |
| Previous use of antibiotics (>2 doses; >5 days before) | 26 | 30.7 |
| Days before | ||
| <10 days | 10 | 11.3 |
| 10–15 days | 6 | 6.8 |
| >15 days | 10 | 11.3 |
| Cephalosporin (2nd, 3rd and 4th) | 8 | 9.1 |
| Carbapenem | 3 | 3.4 |
| Anaerobic antibiotics | 10 | 11.4 |
| Quinolones | 8 | 9.1 |
| Mental confusion or sedation | 14 | 15.9 |
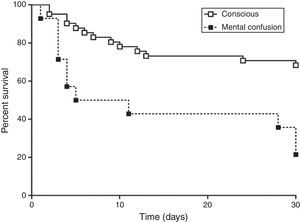
The 30-day mortality was 37.5% with a median survival time of 10 days of hospitalization after bacteremia. Mortality rate was not different with the correct or incorrect therapy. The patients who received correct therapy within 24h did not differ from those who received within 72h, probably due to few patients included in these subgroups. In univariate analysis, chronic renal failure (p<0.001), alteration of mental status (p=0.003), higher leukocytosis (17,215/mm3 and 12,668/mm3, p=0.007), and a higher index of Charlson (≥2, p=0.027) were associated with increased mortality. In multivariate analysis only alteration of mental status was an independent risk factor of mortality. A Kaplan–Meier curve was evaluated for mental status confirming the tendency of higher mortality in the group of patients with this neurological alteration (Fig. 1).
Some conclusions of this study have been described by other authors, such as chronic renal failure.4 Mental status, comorbidities, and leukocytosis are included in the APACHE data, which was not assessed in this study because most patients were not in the intensive care unit. However, in the multivariate analysis, only alteration of mental status was an independent risk. This finding suggests that the infection was severe in these patients, and the encephalopathy can be a marker of mortality among patients with E. coli bacteremia.
Inadequate antibiotic therapy was not associated with increased mortality, probably due to the low mortality rate associated with E. coli in contrast to other gram negative bacilli.5–7 In our analysis, a beta error can explain the lack of difference in mortality in those who received inadequate therapy. The percentage of ESBL-producing E. coli was low (<10%) as previously described in a surveillance program in Brazil.8,9
In conclusion, this retrospective study in one center did not find any significant relationship between mortality and antibiotic therapy adequacy, and mental confusion was a predictor of death in patients with E. coli bacteremia.
Conflicts of interestFelipe F. Tuon received grants from Bayer, Astellas, Merck, Pfizer, Novartis and United Medical (Gilead).
Jaime L. Rocha received grants from Merck, Pfizer, Novartis, Sanofi and AztraZeneca.