Severe fever with thrombocytopenia syndrome (SFTS) associated with severe fever with thrombocytopenia syndrome virus (SFTSV) is an emerging infectious disease. 12 patients with severe fever with thrombocytopenia syndrome in our study were presented mainly with fever and severe malaise. The clinical manifestations typically became worse on the 6th or 7th day. The average fever time is 9.11±1.54 days. Most of them had multiorgan dysfunction, and part of them had hemophagocytic lymphohistiocytosis histiocytosis (HLH). The characteristic laboratory findings in the early stage were the drop of white blood cells (WBC), platelets (PLT) and serum Ca++, while increase of aspartate amino transferase (AST), creatine kinase (CK), and lactate dehydrogenase (LDH). CD3+CD4+ were significantly decreased, while CD3-CD56+ were significantly increased, whereas CD3+CD8+ were constantly elevated throughout the disease course. Ten to 14 days after illness onset, symptoms were improved, accompanied by resolution of laboratory abnormalities. These results indicate that severe fever with thrombocytopenia syndrome has an acute onset and self-limited course. It is a systemic infection. The host immune response caused tissues and organs injury. The improvement of symptoms and laboratory tests is consistent with the elimination of the virus and recover of immune response. Further investigation should be done in order to better understand this disease and guide the clinical treatment.
Accumulating clinical cases with fever, thrombocytopenia and multiorgan dysfunction have been reported in Jiangsu Province of China since middle 1990s.1,2 In 2010, newly emerging patients with the same symptoms in Hubei, Henan provinces and several other provinces were reported,3,4 and then a new virus named severe fever with thrombocytopenia syndrome bunyavirus (SFTSV) was confirmed5 by sequencing the whole genome of the virus. Viruses of the Bunyaviridae family are classified into five genera: Orthobunyavirus, Hantavirus, Phlebovirus, Nairovirus, and Tospovirus. The first four genera can infect mammalian hosts. Most bunyaviruses are transmitted by arthropod vectors such as sandflies, mosquitoes, and ticks, whereas the Hantaviruses are maintained in the natural environment as persistent infections of rodents.6 These viruses can cause hemorrhagic fevers such as Rift Valley fever (RVF), Crimean-Congo hemorrhagic fever (CCHF), hemorrhagic fever with renal syndrome (HFRS), and Sicilian sandfly fever (SFS) in human beings. They may also cause encephalitis and acute respiratory diseases.7 Currently, HFRS and CCHF are endemic in China, and HFRS is an important infectious disease in Jiangsu province.8
Twelve SFTS patients who were admitted to our department from May to August 2011 were enrolled in this analysis. The disease was confirmed according to the Clinical Guidelines on Severe Fever with Thrombocytopenia Syndrome (2010 Edition)9 released by the Ministry of Health of the People's Republic of China. All the data including the clinical data, laboratory findings, imaging results, and outcomes were retrospectively collected by infectious disease physicians. The disease course was divided into four phases: phase1 (days 1–7), phase2 (days 8–10), phase3 (days 11–13), and phase4 (after the 14th day). The changes in serum creatinine, kinases, electrolytes and peripheral blood lymphocyte subsets were monitored and analyzed. All laboratory tests were performed in the First Affiliated Hospital of Nanjing Medical University, except for the real-time PCR to confirm SFTSV RNA from patients in acute stage (within 14 days since onset) that was performed in Jiangsu CDC.
The statistical analysis was performed using SPSS software 17.0 for Windows. Means for continuous variables were compared using independent-group Student's t tests when the data were normally distributed after Kolmogorov–Smirnov test; otherwise, the rank sum test was used. A p-value of <0.05 was considered statistically significant.
These 12 patients (8 men and 4 women) were aged between 43 and 79 years (mean: 64.0 years). Ten patients were farmers living in hilly regions, one was a forest ranger, and the remaining one was a driver who went fishing in an open-water environment four days before disease onset. Five patients believed that they had ever been bitten by ticks. One had diabetes mellitus and one had undergone prostate resection. All these patients were admitted to the hospital at the 3rd to 7th day from onset of illness (median: at the 6th day). The disease onset was acute in all patients. Whole body sore, extreme fatigue, and fever (temperatures of 38°C or higher) were also noted. The clinical manifestations typically became worse on the 6th or 7th day when the temperature reached 40°C. When patients were transferred to our hospital 6–7 days after disease onset, they complained of extreme fatigue (100.0%), dizziness (83.3%) and chest distress (50.0%). Most of them felt abdominal pain (91.7%), diarrhea (91.7%), muscular soreness (75.0%), nausea and vomiting (66.7%). Erosion in the oral cavity (41.7%), mental sluggishness (25%), petechiae and ecchymosis in the skin (33.3%), abdominal tenderness, and superficial lymph nodes (25%) at the same side of the tick-bitted site were also found. Ecchymosis in the skin, and melena and bleeding in the cavity (41.7%) were also observed in patients with severe symptoms. They also presented with wet rales, wheezing sound, hypoxemia, and some nervous system symptoms such as dysphoria, hyperalgia, disturbance of consciousness, hyperspasmia, and status epilepticus. Three patients ultimately died of viral meningoencephalitis, hypoxemia, disseminated intravascular coagulation (DIC), and multiple organ failure. Three of the nine surviving patients persisted with viremia constantly. Viral RNA of one patient was undetectable at day 9 and two at day 10. The body temperature returned normal when virus RNA detection turned out negative, with a temperature recovery time of 9.11±1.54 days.
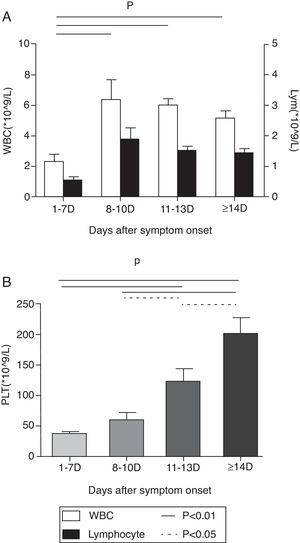
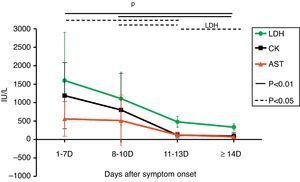
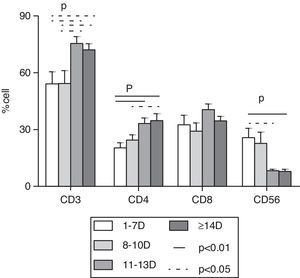
At the first week after disease onset, white blood cell (WBC), lymphocyte and platelet (PLT) counts dropped to the valley levels. Then, at day 8–10, WBC and lymphocyte started to increase, while PLT rose up slowly until day 11–13. The WBC and lymphocyte counts at phase1 were significantly lower than at phases 2, 3, and 4 (p<0.01), while the PLT count at phase 1 was significantly lower than at phases 3 and 4 (p<0.01) and the PLT count at phase 2 was significantly lower than at phase 4 (p<0.01). There was no difference of WBC and lymphocyte counts, but a statistical difference of PLT count was found between phase 2 and phase 3, as well as between phase 3 and phase 4 in pair-wise comparison (p<0.05) (Fig. 1). Urine protein and, microscopically, red blood cells were detected in all 12 patients at the early stage. The average time of urine normalization was 14.11±2.20 days. The LDH, CK, and AST levels remarkably increased at the early stage, and CK and AST remained so for 10 days while LDH returned to normal after 13 days (Fig. 2), in contrast to the ALT level that elevated slightly. BUN and Cr remained normal. Serum Ca++ was reduced until day 13. Compared to those in the mid- to late-stage (day 8–13), CD3+ and CD3+CD4+ were significantly decreased in phase 1 and resumed to increase from phase 3, while CD3-CD56+ were significantly increased in phase1 and started to decrease from phase 3. CD3+CD8+ was constantly elevated in every stage, and there was no statistical difference (Fig. 3). Nine patients received bone marrow examination. Histocytes and phagocytes were occasionally found in one patient and clearly seen in seven patients. The proportion of histocytes or phagocytes ranged 2–11.2%. All patients had active myeloid hyperplasia. Obvious vacuolar degeneration in neutrophils was observed in two patients and inclusion bodies in some neutrophils were found in one patient. The proportion of lymphocytes was low in seven patients, and shaped lymphocytes were observed. Bone marrow culture for bacteria turned out negative.
Change of serum AST, LDH, and CK. LDH, CK and AST levels was increased clearly at the early stage. There is no difference of increasing of LDH, CK and AST levels between phase 1 and phase 2 (p>0.05); but a statistical difference of these changes was found at phase 1, phase 3 (p<0.05) and phase 4 (p<0.01) in pair wise comparison. The statistical difference was also found at phase 2, phase 3 (p<0.05) and phase 4 (p<0.01) in pair wise comparison. However, statistical difference of the change of LDH (p<0.05) was found only at phase 3 and phase 4 in pair wise comparison.
Flow cytometric analysis of peripheral blood lymphocyte subset counts of patients. CD3+ and CD4+ were significantly decreased in phase 1 and started to increase from phase 3, while CD3-CD56+ was significantly increased in phase1 and started to decrease from phase 3. There is a statistical difference at phase 1 and phase 3 (p<0.05), phase1 and phase 4 (p<0.01) of CD3-CD56+. A statistical difference was found at phase 1 and phase 3, phase 1 and phase 4, phase 2 and phase 4 (p<0.05) in pair wise comparison of CD3+ and CD4+. CD3+CD8+ were at high level in every stage, but there was no difference in pair wise comparison (p>0.05).
Patients were treated mainly with symptomatic treatment, supporting therapy such as plasma, platelet, granulocyte colony stimulating factor (GCSF), recombinant human interleukin 11, and gamma globulin. Meanwhile, measures were taken to maintain water and electrolyte balance and to treat complications. Nine SFTS patients (75%) included three patients with hemophagocytic lymphohistiocytosis histiocytosis (HLH) who recovered, while three (25%) patients (aged 58, 70, and 74 years, respectively) ultimately died of viral meningoencephalitis, status epilepticus, coma, hyoxemia and encephaledema at day 8, 9, and 10 day in the course of illness, respectively. Among them, two had HLH.
We herein analyzed 12 patients with SFTSV infection. Most of them lived in hilly areas, and five were bitten by ticks. It seemed that tick bite was the main route of transmission of this disease. Older patients tended to have more severe symptoms perhaps due to their lower immunological capacity to limit the replication of SFTSV. In our series, the disease onset was acute in all the 12 patients, along with diverse clinical manifestations. Most patients developed multiorgan dysfunction, whereas hyperspasmia and obnubilation were seen in critically ill patients. Elevated serum levels of AST, CK, and LDH, as well as urinary protein and red blood cells in the early stage of the disease indicated dysfunction in these organs. One of the characteristic laboratory findings in the early stage was WBC and PLT drop. The major cause of thrombocytopenia may be the enhanced clearance of virus-bound platelets promoted by splenic macrophages.10 Also in our cases, we found that histocyte and phagocyte were present in the bone marrow of most patients. According to the diagnostic criteria for hemophagocytic lymphohistiocytosis histiocytosis (HLH) issued by the American Society of Hematology in 2009,11 five cases could be confirmed as HLH. HLH is a disease characterized by excessive inflammatory response. Although hemorrhagic fever with renal syndrome (HFRS) is a common disease in China, it has been rarely reported that it can lead to HLH. More importantly, considering the high percentage and severity of HLH cases caused by Bunyavirus, it is important to further explore its pathogenesis, clinical treatment and clinical outcome. We found that at the early stage, the amount of NK cells increased dramatically, and then gradually returned to normal after two weeks, while the amount of CD8+T cells increased constantly and did not return to normal in two weeks. It is necessary to do further research to clarify the function of NK cells and CD8+T cells in SFTV infection.12,13 By tracking three patients with viremia, we found that the recovery time of body temperature, as well as other symptoms and laboratory data, are consistent with the presence of viremia.14 This phenomenon was often observed in other six surviving patients. We also observed that the dynamic changes of WBC, lymphocytes, PLT, serum Ca++, LDH, CK, and AST were consistent with the viral replication and the number of Th cells, Tc cells and NK cells, suggesting that viral replication and host immune responses play an important role in determining the clinical outcome. Because of the limited number of cases, further studies with larger number of cases are warranted to investigate the relationship between viremia, host immune status and clinical outcomes. Our study provided detailed clinical analyses of patients infected with SFTSV, as well as laboratory data and immunologic parameters over time. Most SFTS cases experienced an acute, self-limiting disease course and recovered within two weeks if the patients had relatively mild symptoms and no complications. However, a few patients suffered from severe bleeding and central nervous system symptoms and died due to multiorgan dysfunction. Cytokine storm might be in part responsible for the disease progression.15,16 As an emerging infectious disease, clinical types, pathophysiology, pathogenesis, and treatment of SFTS need to be further addressed in future studies.
Conflicts of interestThe authors declare no conflicts of interest.
This study was supported by a grant from Jingsu Province Department of Health (Grant No. H200827) and a grant from Nanjing Medical University (Grant No. NY22229031). We would like to thank Dr. Zhenbao Yu (Canada) for reviewing of the manuscript. We also thank Dr. Longfeng Jiang (Nanjing, China) for collating the data.