Pediatric oncology patients (POP) have a high risk of infections due to impaired immunity. Invasive pneumococcal disease (IPD) is an important cause of severe infection in these patients and it is associated with high mortality. This study aimed to evaluate the incidence and risk factors associated with IPD at a Pediatric Oncology Center in Brazil.
MethodsThis was a retrospective case-control study. All IPD cases in children with cancer from 2005 through 2016 were reviewed. Each case of IPD was matched with two controls from a cohort of patients matched for year of IPD, age and disease in order to assess risk factors. The incidence density was calculated as the number of IPD per 100,000 patients-year.
ResultsA total of 51 episodes of IPD in 49 patients was identified. All pneumococci were isolated from blood cultures. The median age was five years and 67% were male; mortality rate was 7.8%. The IPD incidence density rate in POP was 311.21 per 100,000 patients-year, significantly higher than the rate in the general pediatric population. Severe neutropenia was the only risk factor associated with IPD, after multivariate conditional logistic regression analysis.
ConclusionAlthough pneumococcal disease decreased after the introduction of 10-valent pneumococcal vaccine in the Brazilian national immunization schedule in 2010, there was no decrease in the IPD incidence rate in our cohort. A higher coverage rate of pneumococcal vaccination in children in the general population might be necessary to reduce the incidence rate in this high-risk population.
Streptococcus pneumoniae is a leading bacterial infectious agent in individuals of all ages.1 It is responsible for a wide range of infections and has an extraordinary capability to cause invasive disease with high mortality and morbidity, notably in children younger than 2 and adults older than 65.2
Over recent decades, advances in diagnosis and treatment of pediatric neoplasms led to decreased cancer mortality.3,4 The growing number of patients on treatment or post-treatment emerged as high-risk populations for invasive pneumococcal disease (IPD), due to impaired immune system as a consequence of treatment and underlying disease. Type, timing, duration and intensity of cancer therapy are risk factors for infections, compromising both cellular and humoral immunity.5
Vaccination is the most effective way to protect the general population from IPD. After the introduction of pneumococcal conjugate vaccines (PCVs) into routine immunization programs, many countries have observed a reduction in IPD in the general population.6 In Brazil, the National Immunization Program introduced 10-valent pneumococcal conjugate vaccine (PCV-10) (Synflorix, GlaxoSmithKline) in March 2010 for all children under two years of age. A sharp decrease of IPD has been observed in the target age group.7,8 Nonetheless, there are no data on the impact of PCV-10 in Brazilian children with cancer.
Despite the burden of IPD in children with cancer, there are few published descriptions of the risk factors, clinical presentation, outcomes, incidence rate and impact of PCVs in this risk group. The aim of this study was to evaluate the incidence and risk factors associated with IPD in children with cancer at a Pediatric Oncology Center in Brazil.
Materials and methodsStudy design and populationThis was a retrospective, matched case-control study conducted at the Institute of Pediatric Oncology (IOP/GRAACC) of the Federal University of São Paulo, one of the major pediatric cancer centers in Brazil. IOP/GRAACC is a tertiary care teaching hospital in São Paulo, the largest city in Brazil. It provides care to more than 3700 children with cancer every year, 80% of whom are financed by the public health sector.
We reviewed all episodes of IPD identified in our database between January 1, 2005 and December 31, 2016 in children under 18 years of age diagnosed with cancer or submitted to hematopoietic stem cell transplantation (HSCT). In order to assess risk factors, each case of IPD was matched with two controls from our cohort of patients on the basis of year of IPD, age and underlying disease. Data collected from electronic patient records included sex, age at the diagnosis of IPD, period of treatment, use of antimicrobial prophylaxis and immunosuppressive drugs at the time of diagnosis of IPD, time interval since HSCT, C-reactive protein (CRP) level on admission, immunization, comorbidities (graft versus host disease, anatomical or functional asplenia, hypogammaglobulinemia), and underlying disease. The study was approved by the hospital Clinical Research Ethics Committee.
Case definitionIPD was defined as isolation of Streptococcus pneumoniae from a sterile body site, including blood, cerebrospinal fluid, synovial fluid or pleural fluid. Recurrent IPD was defined as a new isolation of Streptococcus pneumoniae obtained from a normal sterile site ≥30 days after the first isolation.
MicrobiologyPneumococcal identification was performed by the Central Microbiology Laboratory at São Paulo Hospital of the Federal University of São Paulo, using standard microbiological procedures. Isolates of S. pneumoniae were previously stored at −80°C.
Available bacterial isolates from sterile sites were sent for whole genome sequencing (WGS) by Illumina sequencing using the Neoprospecta Microbiome Technologies genomic services (Florianópolis, Santa Catarina, Brazil). The capsular types were determined by the nucleotide sequences of the loccus cps (https://blast.ncbi.nlm.nih.gov/Blast.cgi) (https://www.uniprot.org/) Alleles and multiloccus sequence types (STs) were assigned by using the Streptococcus pneumoniae MLST Databases (http://pubmlst.org/spneumoniae/).
IPD incidence densityInvasive pneumococcal disease incidence density was assessed as the number per 100,000 patients-year. Incidence density was calculated as the number of new cases of IPD occurring in the cohort during a specified year over total persons-year (sum of time periods of each patient with cancer at risk for IPD observed in a given year) X 100,000.
Statistical analysisStatistical analysis was performed using IBM SPSS version 22.0 software (SPSS Inc., Chicago, IL, USA) and Microsoft Office Excel 2016 (Microsoft, Redmond, WA, USA). Descriptive statistics were used to characterize the study population. Chi-Squared test was used to compare characteristics of enrolled children. Univariate and multiple logistic regression analyses were used to calculate odds ratios (ORs) for potential risk factors for IPD in children with cancer. Level of significance was set at p<0.05.
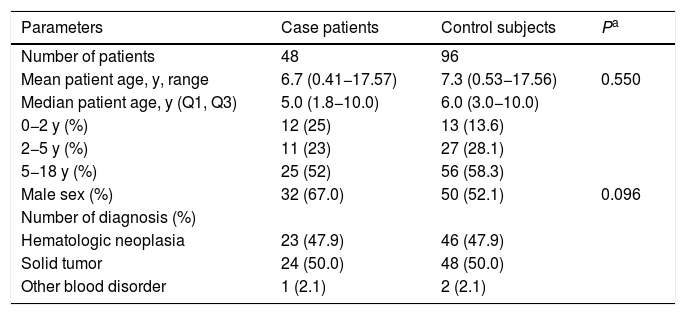
ResultsCharacteristics of cases and risk factor analysisFifty-four episodes of IPD in patients <18 years of age were registered between January 2005 and December 2016. Three of the 54 episodes were excluded from the analysis after confirmation by sequencing that these isolates were two Streptococcus mitis and one Streptococcus oralis. Overall, 51 confirmed pneumococcal isolates were detected in blood cultures from 48 patients (Table 1). The median age of the case patients with the first episode of IPD was five years, 48% were under five years old and 67% were male. Hematologic malignancies accounted for 47.9% (23/48) of all cancers recorded.
Characteristics of 48 children with cancer having their first episode of invasive pneumococcal disease and their 96 paired controls, 2005-2016. Q1 and Q3 indicate first and third quartiles.
| Parameters | Case patients | Control subjects | Pa |
|---|---|---|---|
| Number of patients | 48 | 96 | |
| Mean patient age, y, range | 6.7 (0.41−17.57) | 7.3 (0.53−17.56) | 0.550 |
| Median patient age, y (Q1, Q3) | 5.0 (1.8−10.0) | 6.0 (3.0−10.0) | |
| 0−2 y (%) | 12 (25) | 13 (13.6) | |
| 2−5 y (%) | 11 (23) | 27 (28.1) | |
| 5−18 y (%) | 25 (52) | 56 (58.3) | |
| Male sex (%) | 32 (67.0) | 50 (52.1) | 0.096 |
| Number of diagnosis (%) | |||
| Hematologic neoplasia | 23 (47.9) | 46 (47.9) | |
| Solid tumor | 24 (50.0) | 48 (50.0) | |
| Other blood disorder | 1 (2.1) | 2 (2.1) |
The high proportion of solid tumors in children is due to the fact that IOP/GRAACC is a National Reference Center for solid tumors. One of the patients did not have cancer, he was diagnosed with myeloid aplasia and was submitted to HSCT.
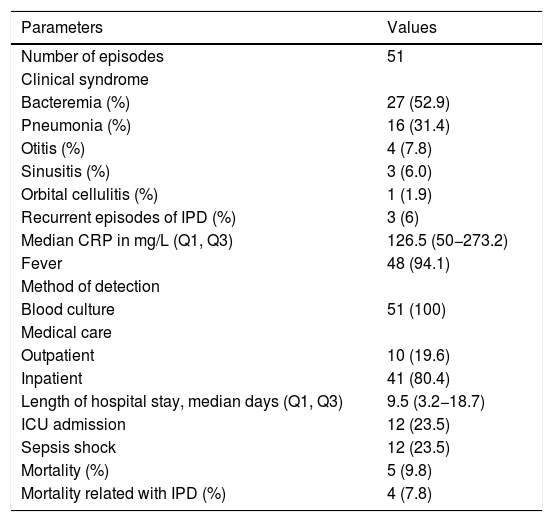
Regarding the clinical presentation, 52.9% (27/51) of the episodes presented with occult bacteremia, 31.4% (16/51) with pneumonia, 7.8% (4/51) with otitis media, and 6.0% (3/51) with sinusitis. One patient was diagnosed with orbital cellulitis (Table 2). There were no cases of meningitis. The majority of the cases presented with fever (48/51) and increased CRP levels with a median of 126.5mg/L (IQR 3.2–18.7). In 80.4% (41/51) of the episodes, patients were hospitalized, with a median length of hospital stay of 9.5 days. Twelve cases (23.5%) required admission to the intensive care unit. None of the 10 children treated without hospitalization had comorbidities and six were receiving outpatient chemotherapy. Ceftriaxone was the initial treatment in 90% of the outpatient group. A patient having palliative care received the combination of amoxicillin and clavulanic acid orally for 10 days, with a favorable outcome.
Clinical presentation, management and outcome of invasive pneumococcal disease (IPD) episodes in children with cancer, 2005–2016.
| Parameters | Values |
|---|---|
| Number of episodes | 51 |
| Clinical syndrome | |
| Bacteremia (%) | 27 (52.9) |
| Pneumonia (%) | 16 (31.4) |
| Otitis (%) | 4 (7.8) |
| Sinusitis (%) | 3 (6.0) |
| Orbital cellulitis (%) | 1 (1.9) |
| Recurrent episodes of IPD (%) | 3 (6) |
| Median CRP in mg/L (Q1, Q3) | 126.5 (50−273.2) |
| Fever | 48 (94.1) |
| Method of detection | |
| Blood culture | 51 (100) |
| Medical care | |
| Outpatient | 10 (19.6) |
| Inpatient | 41 (80.4) |
| Length of hospital stay, median days (Q1, Q3) | 9.5 (3.2−18.7) |
| ICU admission | 12 (23.5) |
| Sepsis shock | 12 (23.5) |
| Mortality (%) | 5 (9.8) |
| Mortality related with IPD (%) | 4 (7.8) |
CRP indicate C-Reactive Protein.
ICU indicates Intensive Care Unit.
Q1 and Q3 indicate first and third quartiles.
The overall mortality rate directly attributed to IPD was 7.8%. One patient who was hospitalized with IPD died 65 days after admission due to a hospital-acquired infection. Out of the four IPD related deaths three occurred in patients with solid tumors (one neuroblastoma, one osteosarcoma, one chordoma) and the remaining had hematologic malignancy (acute myeloid leukemia). Three patients had severe neutropenia with a median neutrophil count of 10 cells/mm3 and the fourth patient was immunosuppressed with high doses of dexamethasone. Three of those four patients were under palliative care, all of whom had comorbidities and died despite the initial therapy of cefepime and vancomycin.
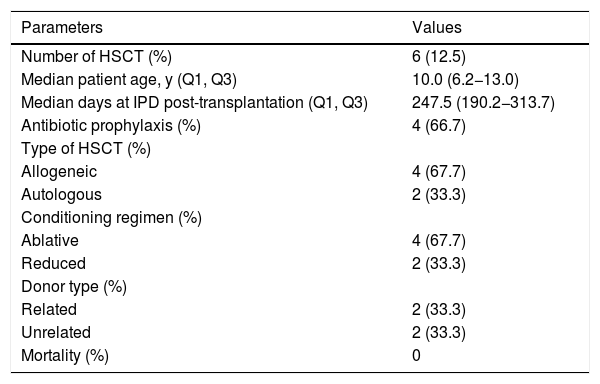
IPD in HSCT patientsSix of the 48 patients identified with IPD in our cohort were hematopoietic stem cell transplant recipients. The median age at the first episode of IPD was 10 years. The proportion of allogeneic stem cell transplantation was 66.7%, half of them with related donors. The median time to IPD onset was 247.5 days (IQR 190.2−313.7) post transplantation (Table 3), with five patients having late onset (>100 days). Only one transplant recipient received one dose of the pneumococcal polysaccharide vaccine (PPSV23) six months after transplantation; the vaccine was administered four months prior to the episode of IPD. Unfortunately, we were not able to identify the pneumococcus serotype. None of the transplanted recipients died from IPD.
Characteristics of the first episode of invasive pneumococcal disease (IPD) in children with cancer who were submitted to hematopoietic stem cell transplantation, 2005-2016.
| Parameters | Values |
|---|---|
| Number of HSCT (%) | 6 (12.5) |
| Median patient age, y (Q1, Q3) | 10.0 (6.2−13.0) |
| Median days at IPD post-transplantation (Q1, Q3) | 247.5 (190.2−313.7) |
| Antibiotic prophylaxis (%) | 4 (66.7) |
| Type of HSCT (%) | |
| Allogeneic | 4 (67.7) |
| Autologous | 2 (33.3) |
| Conditioning regimen (%) | |
| Ablative | 4 (67.7) |
| Reduced | 2 (33.3) |
| Donor type (%) | |
| Related | 2 (33.3) |
| Unrelated | 2 (33.3) |
| Mortality (%) | 0 |
HSCT indicates Hematopoietic Stem Cell Transplantation.
Q1 and Q3 indicate first and third quartiles.
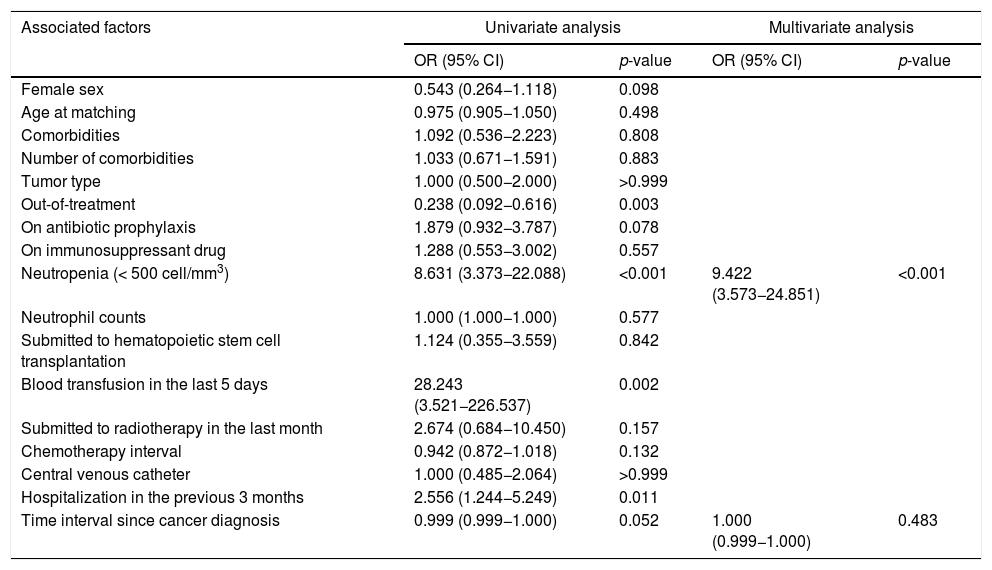
After adjusting for time interval since cancer diagnosis, the only risk factor for IPD in multivariate logistic analysis was neutropenia. Children with neutropenia had a 9.422 higher risk of developing IPD compared with those without that condition (95% Confidence Interval: 3.573–24.851) (Table 4).
Risk factors for invasive pneumococcal disease (IPD) in children with cancer and control subjects.
| Associated factors | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Female sex | 0.543 (0.264−1.118) | 0.098 | ||
| Age at matching | 0.975 (0.905−1.050) | 0.498 | ||
| Comorbidities | 1.092 (0.536−2.223) | 0.808 | ||
| Number of comorbidities | 1.033 (0.671−1.591) | 0.883 | ||
| Tumor type | 1.000 (0.500−2.000) | >0.999 | ||
| Out-of-treatment | 0.238 (0.092−0.616) | 0.003 | ||
| On antibiotic prophylaxis | 1.879 (0.932−3.787) | 0.078 | ||
| On immunosuppressant drug | 1.288 (0.553−3.002) | 0.557 | ||
| Neutropenia (< 500 cell/mm3) | 8.631 (3.373−22.088) | <0.001 | 9.422 (3.573−24.851) | <0.001 |
| Neutrophil counts | 1.000 (1.000−1.000) | 0.577 | ||
| Submitted to hematopoietic stem cell transplantation | 1.124 (0.355−3.559) | 0.842 | ||
| Blood transfusion in the last 5 days | 28.243 (3.521−226.537) | 0.002 | ||
| Submitted to radiotherapy in the last month | 2.674 (0.684−10.450) | 0.157 | ||
| Chemotherapy interval | 0.942 (0.872−1.018) | 0.132 | ||
| Central venous catheter | 1.000 (0.485−2.064) | >0.999 | ||
| Hospitalization in the previous 3 months | 2.556 (1.244−5.249) | 0.011 | ||
| Time interval since cancer diagnosis | 0.999 (0.999−1.000) | 0.052 | 1.000 (0.999−1.000) | 0.483 |
OR indicates odds ratio.
Adjusted OR (95% CI) for conditional logistic regression model.
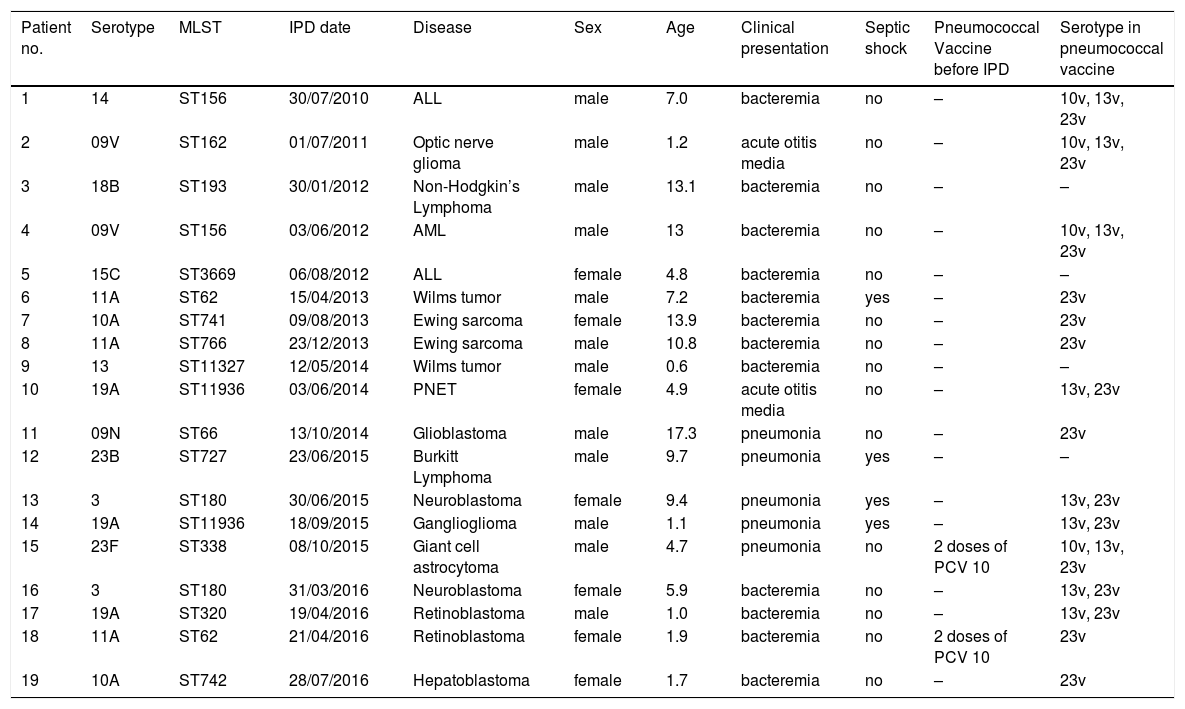
Nineteen isolates of S. pneumoniae were viable to be serotyped using WGS (Table 5). Twelve different serotypes responsible for IPD were identified. Because of the low number of isolates, it was not possible to draw conclusions on the serotype variation over the study period. Nevertheless, one could observe that serotypes 11A, 19A, 10A and 3 were more frequent. ST identified have been previously described in Brazil. Because of lack of information on pneumococcal vaccination in the majority of patients, breakthrough cases could not be identified. Only one patient diagnosed with tuberous sclerosis complex who had an incomplete pneumococcal immunization (two doses of PCV10) had IPD caused by a serotype contained in the PCV10 vaccine. Fifteen STs were identified, including the globally disseminated ST320 (serotype 19A); the STs 62, 180, 156 and 11,936 were observed in two isolates each.
Distribution of serotypes of S. pneumoniae identified and correlation with clinical presentation in children with cancer.
| Patient no. | Serotype | MLST | IPD date | Disease | Sex | Age | Clinical presentation | Septic shock | Pneumococcal Vaccine before IPD | Serotype in pneumococcal vaccine |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 14 | ST156 | 30/07/2010 | ALL | male | 7.0 | bacteremia | no | – | 10v, 13v, 23v |
| 2 | 09V | ST162 | 01/07/2011 | Optic nerve glioma | male | 1.2 | acute otitis media | no | – | 10v, 13v, 23v |
| 3 | 18B | ST193 | 30/01/2012 | Non-Hodgkin’s Lymphoma | male | 13.1 | bacteremia | no | – | – |
| 4 | 09V | ST156 | 03/06/2012 | AML | male | 13 | bacteremia | no | – | 10v, 13v, 23v |
| 5 | 15C | ST3669 | 06/08/2012 | ALL | female | 4.8 | bacteremia | no | – | – |
| 6 | 11A | ST62 | 15/04/2013 | Wilms tumor | male | 7.2 | bacteremia | yes | – | 23v |
| 7 | 10A | ST741 | 09/08/2013 | Ewing sarcoma | female | 13.9 | bacteremia | no | – | 23v |
| 8 | 11A | ST766 | 23/12/2013 | Ewing sarcoma | male | 10.8 | bacteremia | no | – | 23v |
| 9 | 13 | ST11327 | 12/05/2014 | Wilms tumor | male | 0.6 | bacteremia | no | – | – |
| 10 | 19A | ST11936 | 03/06/2014 | PNET | female | 4.9 | acute otitis media | no | – | 13v, 23v |
| 11 | 09N | ST66 | 13/10/2014 | Glioblastoma | male | 17.3 | pneumonia | no | – | 23v |
| 12 | 23B | ST727 | 23/06/2015 | Burkitt Lymphoma | male | 9.7 | pneumonia | yes | – | – |
| 13 | 3 | ST180 | 30/06/2015 | Neuroblastoma | female | 9.4 | pneumonia | yes | – | 13v, 23v |
| 14 | 19A | ST11936 | 18/09/2015 | Ganglioglioma | male | 1.1 | pneumonia | yes | – | 13v, 23v |
| 15 | 23F | ST338 | 08/10/2015 | Giant cell astrocytoma | male | 4.7 | pneumonia | no | 2 doses of PCV 10 | 10v, 13v, 23v |
| 16 | 3 | ST180 | 31/03/2016 | Neuroblastoma | female | 5.9 | bacteremia | no | – | 13v, 23v |
| 17 | 19A | ST320 | 19/04/2016 | Retinoblastoma | male | 1.0 | bacteremia | no | – | 13v, 23v |
| 18 | 11A | ST62 | 21/04/2016 | Retinoblastoma | female | 1.9 | bacteremia | no | 2 doses of PCV 10 | 23v |
| 19 | 10A | ST742 | 28/07/2016 | Hepatoblastoma | female | 1.7 | bacteremia | no | – | 23v |
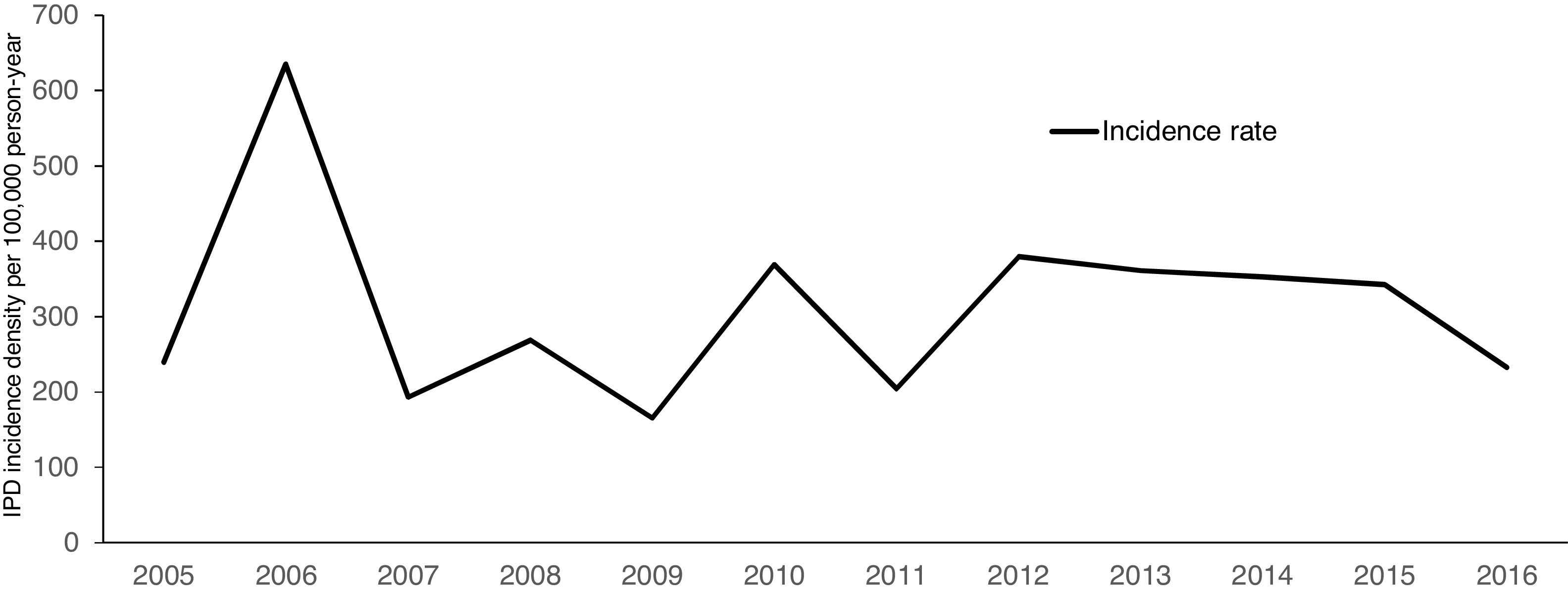
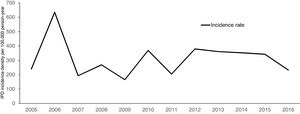
Over a 12-year period, the IPD incidence density in the analyzed cohort varied with time, without declining (Fig. 1). The highest incidence was observed in 2006, with 635.39 cases per 100,000 persons-year. The lowest incidence density of the period was observed in 2009, one year before the introduction of PCV 10 (165.16 per 100,000 persons-year). The cumulative incidence in the period of analysis was 311.21 per 100,000 persons-year.
DiscussionThis study described the epidemiology of IPD in children with cancer at a major pediatric cancer center in Brazil over 12 years. The IPD incidence density rate in POP was 311.21 per 100,000 patients-year, significantly higher than the rate in the general pediatric population. By contrast, the mortality rate was low. Severe neutropenia was the only risk factor associated with IPD. Although pneumococcal disease decreased after introduction of the 10-valent pneumococcal vaccine in the Brazilian national immunization schedule in 2010, there was no decrease in IPD Incidence rate in our cohort.
It is well known that IPD has a high burden in children younger than two years, representing 57% of the cases in Americas and 56% in Europe.9 In our study, this population remained affected (25%), but a high proportion (52%) of IPD was observed in patients over five years.
A longitudinal study at a New York center observed a decline in IPD in children younger than five years after the introduction of PCV-7 and PCV-13.10 In a cohort of children with acute lymphoblastic leukemia, the median age at IPD onset was six years, almost the same age found in our study.11 There was a tendency to more IPD cases among males (67%) (p=0.096), as previously reported.12
Occult bacteremia was the most common clinical presentation (52.9%). That represents a major diagnostic challenge in patients with cancer. In one study, occult bacteremia represented 65% of the cases in transplant recipients.13 Fever was a frequent life-threatening sign (48/51) extremely important in oncologic patients with IPD, indicating the need for blood culture and risk stratification to an early administration of broad-spectrum antibiotics.14 Overall, a low mortality rate (7.8%) was observed, possibly because all patients with fever received antibiotics in the first hour and no meningitis was identified. The percentage of hospitalization was lower (80.4%) than previously reported (91.3%).15
In multivariate analysis, neutropenia was the only risk factor associated with IPD, with an OR of 9.4 (95%CI 3.6–24.8). In patients with cancer, severe and persistent neutropenia is an important risk factor, especially for fungal and bacterial infections.16 Febrile neutropenia is an oncologic emergency, and early administration of broad-spectrum antibiotics is necessary for a favorable outcome. Patients with cancer who are receiving high-dose chemotherapy or radiation therapy have a functional impairment of neutrophils. In our cohort, the median time interval between chemotherapy initiation and IPD was 11 days (IQR 7–13), which is when the number of neutrophils is usually at the lowest level.
In children with underlying chronic diseases, hematologic malignancies appeared as a high IPD risk group with an adjusted OR of 52.1 (95%CI 13.7–198.2).17 By contrast, there were no differences in the risk of IPD in patients with other chronic conditions such as congenital forms of immune deficiency, renal disease, or heart disease.17
The majority of cases of IPD occurs after 100 days following transplant in adults submitted to HSCT.18 Pediatric patients appear to follow a similar presentation. Olarte et al. reported a median time to IPD post-transplant of 11.1 months. Among HSCT and solid organ transplantation (SOT), Schutze et al. observed a median time of 14 months.13,19 In our study, the median time to IPD onset was approximately 8.25 months post transplantation. None of the patients submitted to HSCT died. In this group, the initial antimicrobial therapy included a 4th generation parenteral cephalosporin or a carbapenem combined with vancomycin.
To understand the burden of the disease in children with cancer, we estimated the IPD incidence density over 12 years in our cohort. IPD incidence decreased in the general population after the introduction of pneumococcal conjugate vaccines (PCVs) in many countries. In Brazil, three years after PCV-10 introduction, a reduction of 44% in IPD rates (95% CI 15.8–72.5) was observed in children aged 2–23 months. No effect was seen in unvaccinated age groups.7 In a review that examined the impact of PCV-10 in Brazil after five years8, a small number of IPD were observed in many reports, with a reduction in the incidence rate of pneumococcal meningitis in under 2-year-olds.20
In our study, there was no trend towards a decline in IPD incidence rate. The overall cumulative IPD incidence density in 12 years was 311.21 cases per 100,000 persons-year, which was an extremely high incidence, comparable to IPD incidence of HIV-infected individuals who were not on antiretroviral therapy (281 per 100,000 patients)21 and higher than the incidence in patients submitted to transplantation. We did not find any report on IPD in children with cancer in Brazil.
Shigayeva et al. observed that IPD incidence rate decreased from 14.1 to 4.9 per 100,000 among immunocompetent children 10 years after PCV-7 introduction in Toronto. However, IPD incidence rate among immunocompromised children did not change significantly, 112 and 199 per 100,000 (IRR 1.78; 95% CI, 0.53–5.98).22
A divergent trend was perceived in a retrospective study at one center in the United States,10 where 343 IPD cases in patients with cancer over 20 years were reviewed. In patients with <5 years, there was a 79% decrease in IPD after PCV-7 introduction, from 11.2 per 1000 patients visits to 2.38 per 1000 patients visits. In another American study with pediatric transplant recipients, the incidence rate was 127.2 cases per 100,000 children per year for pediatric solid organ transplantations.13 Tran et al.23 reported an IPD incidence of 176 per 100,000 children per year among solid organ transplant recipients.
A possible reason for the maintained incidence density of IPD after PCV-10 introduction is that the vaccine has some limitations, as it is available for children up to five years of age. When the vaccine was introduced in Brazil, it was available only for children under two years of age. Many patients diagnosed with cancer before the introduction of PCV-10 (19/48) were not eligible for the vaccine. Moreover, some patients could not complete the recommended schedule due to interruption of immunization when chemotherapy was initiated. In our cohort, only four patients were vaccinated with any dose of a pneumococcal vaccine before IPD.
Despite high vaccine coverage rates in infants (81.7% in 2011, and 94.2% in 2015),24 the introduction of PCV-10 had a small impact in reducing IPD incidence rate in children over five years in Brazil and no effect in unvaccinated age groups.7 This group represents a large proportion of patients who were diagnosed with IPD during the period of analysis. More time might be necessary to allow a direct effect from PCV-10 introduction in older children.
Moreover, any possible indirect and direct protective effect of PCV-10 in children with cancer is reduced by the replacement of serotypes responsible for IPD.25 The emergence of serotypes not contained in PCV-10 has been described in some reports, especially 19A, 22F and 3. Three to five years after PCV-10 introduction, serotypes 22F and 19A were the most frequent serotypes isolated from elderly patients with IPD in Southern Brazil.26 The incidence of serotype 19A increased worldwide with the emergence of the clone ST320, identified in our cohort. ST320 is associated with penicillin resistance27,28 and it seems to have advantages on nasopharynx colonization over other serotypes.29 In a study in Rio de Janeiro, Brazil, researchers evaluated pneumococcus isolated from patients with cancer. PCV-10, PCV-13, and PPSV-23 had an estimated coverage of capsular types of 34%, 50% and 68%, respectively.30 Considering that vaccine effectiveness in patients with cancer is lower than in the general population, an improved vaccine that covers more serotypes is needed.
In 2019, PCV-13 was introduced in Brazil for patients after the diagnosis of underlying conditions such as HIV infection, HSCT and SOT recipients, as well as oncologic patients.31 Although a better vaccine immune response could be achieved if children vaccinated before cancer diagnosis, this new immunization guidelines might alter the scenario of IPD in children with cancer in the future.
This study has some limitations: due to its retrospective nature, we could not determine all the serotypes that caused IPD, what may have also pointed out some streptococcal isolates that could belong to another species. On the other hand, this study is important due to the lengthy period of inclusion of patients and the high-risk population evaluated.
In conclusion, we described IPD clinical presentation and outcomes in children with cancer in a tertiary pediatric cancer center in Brazil. Neutropenia was the only risk factor associated with IPD. Over 12 years, the incidence density of IPD in oncologic children remained very high. PCV-10 introduction did not reduce IPD incidence in our cohort. In addition to a high coverage rate of pneumococcal vaccination in children in the general population, the recent introduction of PCV-13 in oncologic patients might help reduce the high incidence rate in this high-risk population.
Conflicts of interestThe authors declare no conflicts of interest.
Data availabilityThe data that support the findings of this study are available from the corresponding author upon reasonable request.
The authors received an Investigator Sponsored Research Grant from Pfizer. Pfizer had no involvement in the study design, in the sample collection, analysis and interpretation of data, in the writing of the report or in the decision to submit the article for publication.