The aim of the present study was to investigate the association between polymorphism in the interleukin-10 gene promoter at position −1082 in human immunodeficiency virus-infected patients who had presented allergic reaction due to efavirenz. The study included 63 patients treated at the Hospital São José de Doenças Infecciosas, Fortaleza, Ceará, Brazil. Twenty-one patients who had presented allergic reaction to efavirenz were compared to 42 patients with no allergic reaction following exposure to this drug. Blood samples were collected for DNA extraction and submitted to the restriction fragment length polymorphism – polymerase chain reaction technique. The −1082AA genotype was significantly more frequent in allergic patients as compared to non-allergic patients (p=0.019; χ2=5.534; OR=3.625; 95% CI=1.210–10.860). Likewise the allele IL-10 −1082A was identified significantly more often among efavirenz allergic patients than in the non-allergic group (p=0.009; χ2=6.787; OR=3.029; 95% CI=1.290–7.111). These findings suggest that the polymorphism in the interleukin-10 gene promoter −1082G/A can be related to the development of allergic reactions to efavirenz.
According to the World Health Organization (WHO), the term adverse drug reaction (ADR) can be defined as any noxious or unintended reaction that appears after drug administration at standard doses, for the purpose of prophylaxis, diagnosis and treatment of a disease.1 Clinical manifestations of adverse reactions to antiretroviral drugs can affect individuals on various levels of severity. The most common ADRs occur early in therapy and include gastrointestinal effects, such as nausea, bloating, and diarrhea, which may be transient or persistent. Less common reactions include Zidovudine AZT-associated anemia and hypersensitivity to non-nucleoside reverse transcriptase inhibitors (NNRTIs).2 It is known that viral infections, such as those caused by Epstein–Barr virus (EBV) and human immunodeficiency virus (HIV), constitute increased risk factors for the development of drug hypersensitivity. In the case of HIV, studies have shown that the frequency of drug hypersensitivity is estimated to be in the range of 3–20%, and that skin rashes related to drugs are 100 times more frequent in HIV-infected patients than in the general population.3 The main adverse effects associated with efavirenz, a drug which belongs to the NNRTI class, are due to disorders of the central nervous system (CNS), such as dizziness, drowsiness, headache, inability to concentrate, nightmares, and depression. These effects can be observed in approximately 40% of patients in the early days or weeks of treatment.4 In addition to these effects, it is reported that drug rashes occur in 27% of the adults and 45% of the children, occurring around the second week of treatment.5 The increased frequency of drug allergy in HIV patients can be attributed to immune system dysregulation or vulnerability to oxidative stress. Drug exposure is a necessary component, but not the only causative agent that induces drug allergy. Allergy also depends on individual susceptibility. In this way, individual susceptibility is thought to be multifactorial, including genetic factors.3,5 Some studies of genetic predisposition to antiretroviral allergy have shown a strong association between the presence of HLA-B* 5701 and hypersensitivity to abacavir.6,7 Thus, screening for this allele before the initiation of antiretroviral therapy could be important in the identification of patients at increased risk for allergic reaction to abacavir.8 Other genetic studies have shown that polymorphism of genes that regulate cytokine production can affect the individual immune response.9
Interleukin-10 (IL-10) is an important anti-inflammatory cytokine secreted by various cells of the immune system, including T lymphocytes, macrophages, dendritic cells and monocytes. This cytokine has immunoregulatory effects such as inhibition of pro-inflammatory cytokines IL-1, IL-6, IL-12, IL-18 and tumor necrosis factor TNF, as well as co-stimulatory molecules on antigen-presenting cells.10 Single nucleotide polymorphisms (SNP) at positions −1082 (A/G), −819 (T/C), −592 (C/A) of the proximal promoter region of the IL-10 gene may affect the cytokine in vitro production.11,12 The presence of the A allele at position −1082 seems to be associated with low levels of IL-10 production, and occurs independently of polymorphisms at other positions.11 The aim of the present study is to investigate the association between allergic reaction to efavirenz and polymorphism in the promoter region of the IL-10 gene at position −1082G>A in HIV-infected patients.
This is a case control study involving 63 HIV-infected adult patients on Highly Active Antiretroviral Therapy (HAART) receiving care at Hospital São José Infectious Diseases in Fortaleza, Ceará, Brazil. Case definition of drug allergy to efavirenz included rash, skin eruption, urticaria, and/or erythema following exposure to efavirenz. Additionally, efavirenz withdrawal led to a complete remission of the allergic reaction.
The assistant physicians of patients suspect of having an allergic reaction to efavirenz were to complete a drug change/modification request form (ADR reporting inform) in order to discontinue efavirenz and switch to another regimen. From 2006 to 2012, there were 84 patients with drug reaction reporting forms who were contacted over telephone calls and invited to participate in the study. Of those, only 21 eligible patients were located and agreed with blood collection. There were nine males and 12 females, aged 24–71 years. The control group consisted of 32 males and 10 females, aged 20–67 years, HIV-infected patients on HAART-containing efavirenz for at least six months without any evidence of adverse cutaneous reactions to the drug. All the participants signed a written informed consent. The project was approved by the Ethics Committee in Research, Hospital São José de Doenças Infecciosas, number 025/2011.
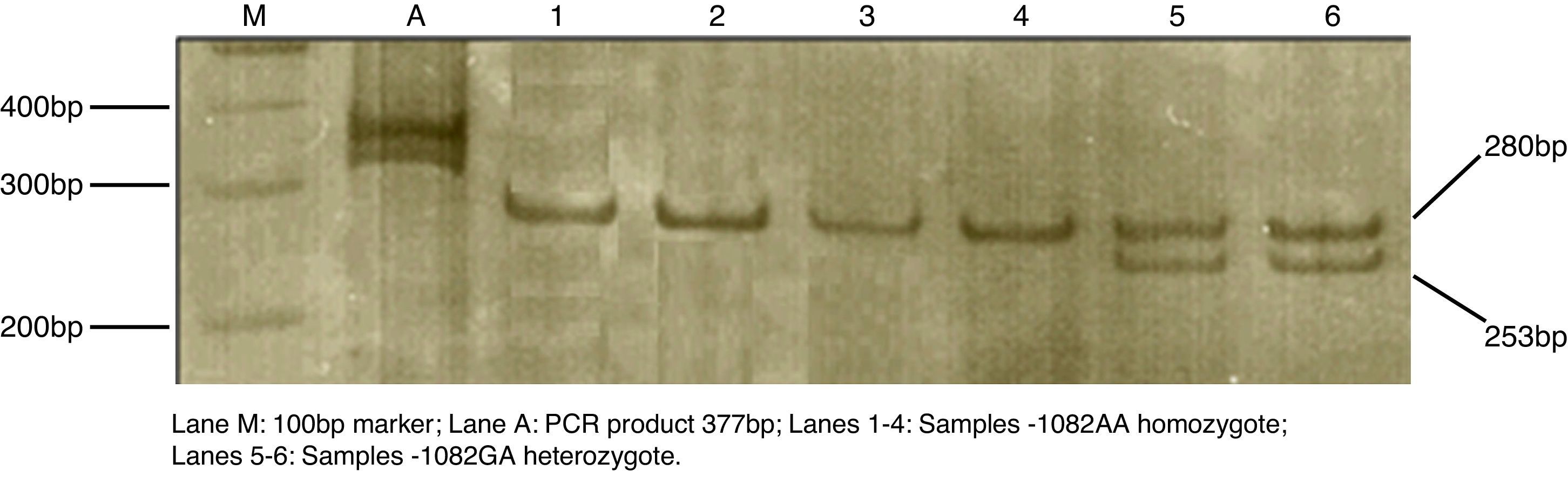
Venipuncture of 4mL of whole blood was collected in a tube containing ethylenediaminetetraacetic acid EDTA as an anticoagulant. DNA was extracted using a commercial extraction kit, Biopur Extraction Kit Mini Plus Spin – 250 (Biopur, Brazil), according to the manufacturer's instructions. Polymorphism of IL-10 (−1082G>A) was detected by PCR-RFLP, as previously described by Koch et al.13 The reaction was performed in a total volume of 20μL reaction solution, using TopTaq Master Mix (Qiagen, Germany) with addition of 0.01% bovine serum albumin (BSA), primer at concentration of 20pmol/μL and approximately 25ng of DNA template. The polymerase chain reaction (PCR) was performed in a thermocycler (Eppendorf, Germany) under the following conditions: initial denaturation at 94°C for 4min, followed by 35 cycles of 94°C for 40s, 56°C for 35s and 72°C for 40s. The final extension was done at 72°C for 5min. The PCR products of 377bp were then digested with restriction endonuclease XagI (Fermentas, Lithuania) at 37°C for 16h. The 280 and 97bp fragments corresponded to the −1082A allele and 253, 97 and 27bp to the −1082G allele. The analysis of the digestion products was done by electrophoresis on 6% polyacrylamide gel after silver staining (Fig. 1).
The statistical analyses were performed using the commercial software GraphPad Prism version 5.00 for Windows, (GraphPad Prism, Inc., La Jolla, CA, USA). The Chi-square test was used to test for the association between efavirenz allergy and genotypic and allelic frequencies. Odds ratio (OR) with a 95% confidence interval was calculated. A p-value<0.05 was considered of statistical significance.
In the analysis of the allele frequency, a statistically significant difference was found comparing case and control groups (p=0.009, Table 1). A higher frequency of the A allele was found in the efavirenz-allergic group. The genotype AA at the position −1082 for IL-10 was much more frequent among efavirenz-allergic patients than in the non-allergic group (p=0.019, Table 1). The OR indicated that the probability that an individual would present an allergic reaction to the drug was 3.625 times greater when the in case of AA genotype. AA genotype was identified in 62% of efavirenz-allergic patients. On the other hand, the genotype GG or GA at the position −1082 for IL-10 was identified in 69% of non-allergic patients. According to Turner et al.,11 the carriage of the AA genotype at position −1082 is one of the factors which determines lower levels of IL-10. Our results demonstrated that individuals with the presence of the −1082A allele and the −1082AA genotype are more prone to suffer allergy to efavirenz.
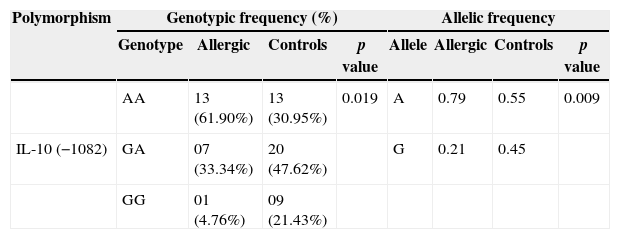
Genotypic and allelic frequencies of IL-10 −1082G>A among patients who presented allergy to efavirenz and among controls.
| Polymorphism | Genotypic frequency (%) | Allelic frequency | ||||||
|---|---|---|---|---|---|---|---|---|
| Genotype | Allergic | Controls | p value | Allele | Allergic | Controls | p value | |
| AA | 13 (61.90%) | 13 (30.95%) | 0.019 | A | 0.79 | 0.55 | 0.009 | |
| IL-10 (−1082) | GA | 07 (33.34%) | 20 (47.62%) | G | 0.21 | 0.45 | ||
| GG | 01 (4.76%) | 09 (21.43%) | ||||||
Genotypic frequency: χ2=5.534; odds ratio (OR)=3.625; 95% confidence interval (CI)=1.210–10.860.
The p-value for genotype frequency is calculated by IL-10 −1082AA vs IL-10 −1082GA/GG. Allelic frequency: χ2=6.787; OR=3.029; 95% CI=1.290–7.111.
It is observed that some patients treated with various drugs may exhibit variability in therapeutic response to the drug and may have susceptibility to some adverse reactions. Pharmacogenetics is gaining wide applicability in clinical pharmacology once the study of genetic factors may contribute to the effectiveness and safety of drug therapy.14,15
Saag et al.7 evaluated the detection of HLA-B*5701 as a marker for hypersensitivity to abacavir after confirmation of drug allergy by patch testing in Black and Caucasian patients living in the United States. The results of the survey showed 100% sensitivity for HLA-B*5701 as a marker of drug allergy to abacavir in both groups. Some other studies suggest that screening for HLA-B*5701 should be performed before starting the abacavir therapy, thereby reducing the risk of allergic reactions to this drug.8
Several studies associating polymorphism in the promoter of IL-10 and increased pathogenesis in some infectious diseases have been carried out. Patients with toxoplasmic retinochoroiditis were more frequently carriers of the −1082A allele (AA+GA genotypes) than controls. It was also suggested that the genotype GA was associated with toxoplasma retinochoroiditis.16 In another study, it was found that the polymorphism in the promoter of IL-10 (−1082G>A) was associated with the development of cardiomyopathy in individuals with Chagas disease. Furthermore, this study could demonstrate that low expression of the cytokine was associated with worse cardiac function.17
The association of the polymorphism of IL-10 with hypersensitivity reactions to drugs is promising. A study of polymorphisms in the promoter of IL-10 at the positions −819 and −592 was conducted in the French population. It was found that atopic women presenting the genotypes CT or TT at the position −819 and the genotype CA or AA at the position −592 had higher risk for developing immediate hypersensitivity reaction to β-lactams. This finding is relevant because the alleles −819 T and −592 A are associated with lower expression of IL-10. In regard to type I hypersensitivity, as IL-10 has an immunomodulatory activity, its reduction enables the Th2 profile, with consequent stimulation of IL-4, IL-9, and IL-13. These cytokines favor the production of IgE which culminates with immediate hypersensitivity.18
Qiao et al.19 observed an association between levels of specific IgE, cytokines and polymorphisms of IL-4 C/T and IL-4RαQ576R in patients with penicillin allergy. According to the authors, the allele IL-4Rα*Q576 was present more frequently in allergic patients than in control subjects. Furthermore, it was demonstrated that the allele IL-4Rα*Q576 was associated with the expression of some types of penicillin-specific IgE, which included benzylpenicilloyl, phenoxomethylpenicillanyl and ampicillanyl.
The different forms of exanthems (rashes) are the most frequent drug reactions. Some appear rapidly after drug intake, for example, immediate-type reactions like urticaria and angieodema. Others appear hours or days after drug intake; they are the so-called delayed-type reactions and comprise a broad spectrum of clinical and distinct histopathological features.20 In our study, the high frequency of −1082AA genotype in the IL-10 gene promoter is probably enabling TCD4 Th1 and/or Th2 profiles. Reduction of IL-10 levels promotes increased activity of CD4+ T lymphocytes of the Th1 and Th2 profiles, which would produce a greater quantity of IFN-γ and IL-4 respectively.12
Some limitations of our study are related to the high of refusal rate of patients to join the study. Various factors appear to be relevant, such as low socioeconomic status, distance from the patient's residence to the hospital, inability to understand the purpose of the study, fear of exposing his (her) disease status to others. We could not perform skin tests to confirm drug allergy to efavirenz because of logistical reasons [several readings in the case of epicutaneous tests (24h, 48h and 72h)].
Our data could suggest that polymorphisms in regulatory regions of cytokine production are among the factors that may contribute to an individual susceptibility to drug allergy. Other studies are necessary to prove our findings.
Conflicts of interestThe authors declare no conflicts of interest.
This study was financially supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, process 554970/2010-4).