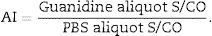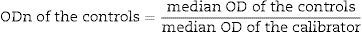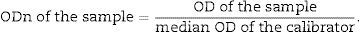The aims of this study were to compare the automated AxSYM avidity assay index with the BED capture enzyme immunoassay test and to calculate the HIV-1 incidence using the BED capture enzyme immunoassay and AxSYM avidity assay index algorithms within a population seeking the Voluntary Counselling and Testing Centres in two municipalities in the Metropolitan Region of Recife, Northeast of Brazil. An analysis was conducted in 365 samples that tested positive for HIV infection from frozen serum collected during the period 2006–2009. There was a similar proportion of males and females; most patients were heterosexual (86%) with a median age of 29 years. Of the 365 samples, 102 (28%) and 66 (18.1%) were identified as recent infections by BED capture enzyme immunoassay and AxSYM avidity assay index, respectively. The HIV-1 total incidence in the BED capture enzyme immunoassay and AxSYM avidity assay index algorithms were: 0.79 (95% CI: 0.60–0.98) and 0.34 (95% CI: −0.04 to 0.72), respectively. Incidence was higher among men. There was good agreement between the tests, with a kappa of 0.654 and a specificity of 95.8%. AxSYM avidity assay index may be helpful in improving the quality of the estimates of recent HIV infection and incidence, particularly when used in a combined algorithm with BED capture enzyme immunoassay.
Brazil has reported 656,701 cases of AIDS, and in recent years there has been an increase in the rate of cases in the North and Northeast regions of the country, when compared to the South and Southeast regions. Furthermore, the Northeastern state of Pernambuco and its capital Recife present the highest rates of new cases of AIDS of any state or state capital in Northeast Brazil.1
The growing interest in estimating HIV-1 incidence has led to the development of a variety of laboratory assays. This methodology is used only for epidemiological investigation purposes, and is referred to by the recently introduced term RITA (Recent Infection Testing Algorithm), used to describe laboratory assays that are able to identify a transitional period of recent seroconversion of HIV infection.2
In 1998, an adaptation of a diagnostic test for HIV-1 that would revolutionize epidemiological methods through its ability to identify recent HIV-1 infection was described in the literature.3 The test differentiated recent infections (less than six months) from established infections (more than six months), due to the kinetics of antibodies after the onset of HIV-1 infection. This test allowed to estimate HIV-1 incidence from cross-sectional studies, using a single serum sample per patient.
The BED capture enzyme immunoassay (BED-CEIA), the assay for HIV-1 incidence, has peptides as antigens derived from the immunodominant region of gp41, containing sequences of subtypes B, E and D, which are representative of multiple subtypes of HIV-1. The time at which HIV infection occurred can be estimated, given that the ratio between the total IgG and anti-HIV IgG varies according to the progression of the disease.4 This technique has been widely used in conjunction with mathematical tools for estimating the incidence of HIV infection.5
Another method for distinguishing between recent and established HIV infections based on antibody avidity (affinity) was described in 2002.6 This methodology is based on the principle that the antibody avidity produced in the initial phase of the infection (within six months of seroconversion) is low, in contrast to that observed in established infections (more than six months).6 Besides the simplicity and automated nature of the technique, in theory, it would also be able to detect a high proportion of subtypes of HIV-1 and would not be affected by the stage of the infection. The association of the two tests has been used to confirm the positive results of BED, resembling results of incidence data of cohorts that have documented seroconversion.7
In view of the importance of comprehending the dynamics of the HIV epidemic, the aim of this study were to compare the magnitude of agreement between the automated test AxSYM avidity assay index (Ax-AI) with the BED-CEIA test and to calculate the HIV-1 incidence using BED and Ax-AI within a population seeking Voluntary Counselling and Testing Centres (VCTs) in two municipalities of the Metropolitan Region of Recife, in Northeast Brazil.
Frozen samples from patients diagnosed with HIV infection during the period 2006–2009 were obtained from two VCTs in the municipalities of Cabo de Santo Agostinho and Paulista, in the Metropolitan Region of Recife – Northeast Brazil. Three hundred and eighteen patients were diagnosed as HIV-infected at the VCT in Cabo de Santo Agostinho, and aliquots from 206 (64.8%) of them were available for further testing. At the Paulista VCT, 179 cases of HIV infection were detected, but only 159 (94%) had serum available for testing. Thus a total of 365 serum samples were analyzed.
Samples from patients with previous use of antiretroviral therapy (ART), infected through vertical transmission, and those with previous diagnosis of HIV infection occurring more than six months prior to the collection of the sample were excluded.
Data relating to the individuals’ gender, age, and sociodemographic data were retrieved from the databank of the HIV test result information system (SIREX-HIV) at the VCTs. This study was approved by the Research Ethics Committee of the Hospital Agamenon Magalhães (protocol number 518/2009).
For the Ax-AI the sample was defrosted, two aliquots of 20μL were obtained and diluted 1:10. One aliquot (PBS aliquot) was diluted with 180μL of phosphate-buffered saline (PBS) and the other (G aliquot) was diluted with 180μL of 1M guanidine solution (G) (Sigma–Aldrich). After dilution, all samples were vortex-stirred and incubated for 10min at room temperature. The two aliquots of each sample were tested using the automated AxSYM HIV-1/2 gO assay (Abbott Diagnostics Division, Wiesbaden-Delkenheiem, Germany), in accordance with the manufacturer's instructions.6
Addition of a denaturing agent (guanidine) breaks hydrogen bridges, which helps determining the secondary structure of the antibodies and leads to a residual effect from the antigen–antibody interaction. This procedure has greater effect on recent antibodies, which are low-avidity antibodies.8 This procedure results in an S/CO ratio that is lower for the G aliquot than for the PBS aliquot. The avidity index (AI) is calculated after obtaining the S/CO ratios for the PBS and G aliquots as follows:6,9
A cutoff value of 0.8 distinguishes an infection of less than six months6,10 from a long-term infection (a duration of more than six months).
The BED-CEIA test (Calypte Biomedical Corporation, Oregon, USA) is an IgG-capture ELISA technique based on detecting increasing proportions of specific anti-HIV-1 IgG following seroconversion. Anti-HIV IgG and non-specific IgGs are captured in the solid phase of the microplate. After incubation and washing, peptides from the immunodominant region of gp41 that contain sequences of the subtypes B, E and D were added. After further incubation and washing, a streptavidin conjugate was added, followed by an incubation period of 90min. The response is ascertained after the addition of tetramethylbenzidine (TMB) with a spectrophotometer (wavelength 450nm).4,11
Following the immunoassays and reading of the spectrophotometer, the normalized optical densities (ODns) were obtained, as follows:
The tests were interpreted as follows: all samples with ODn≤1.2 were tested in triplicate to confirm whether it was a recent or long-term infection. Confirmation interpretation: samples with OD>0.8 were characterized as long-term seroconversion and those with OD≤0.8 as recent seroconversion.12
HIV-1 incidence and 95% confidence interval (95% CI) for the BED-CEIA and Ax-AI algorithms were calculated using the spreadsheet provided by the South African Centre for Epidemiological Modelling and Analysis (SACEMA) (http://www.incidence-estimation.com/page/tools-for-incidence-from-biomarkers-for-recent-infection).13 The following parameters were reported on the spreadsheet: the Estimated Mean Duration of Recent Infection (MDRI), which was 180 days for the Ax-AI and 155 days for the BED-CEIA;4 the estimated False Recent Rate (FRR%), which was 5.6%,14 and 10.6% for the BED-CEIA and Ax-AI, respectively;7,14 number of HIV-positive individuals; number of HIV-negative individuals and number of recent HIV-1 infections. Incidence estimates were expressed as the number of new HIV infections per 100 person-years.
Sensitivity, specificity, positive and negative predictive values, accuracy, likelihood ratios for positive and negative tests, and kappa were obtained by comparing the results of Ax-AI to those of BED-CEIA, using the MSOffice Excel version 2010 (Microsoft Corporation) and the SPSS for Windows version 12.0. Significant associations between the variables were considered when p-values were less than 0.5.
The study population consisted of 177 men (48.5%) and 188 women (51.5%), of whom 53 (28.2%) were pregnant. The median age was 29 years. Other epidemiologic and behaviour data are listed in Table 1. Ax-AI and BED-CEIA results were compared using 365 samples. One hundred and two samples were identified as recent infection by BED-CEIA, and 77 samples by Ax-AI. For the status of long-term infection, the BED-CEIA and Ax-AI identified 263 and 288 samples, respectively. Both tests were concordant in 66 samples classified as recent infection and 252 as a long-term infection.
Demographics and behavioural data of patients newly diagnosed as HIV-positive at two VCTs in Pernambuco – Brazil, 2006–2009.
| Variables | HIV-positive patients |
|---|---|
| Gender | |
| Male | 177 (48.5) |
| Female | 188 (51.5) |
| Pregnant | |
| Yes | 53 (28.2) |
| No | 135 (71.8) |
| Median age (years) | 29 |
| Sexual orientation | |
| Heterosexual | 304 (86) |
| MSMa | 50 (14) |
| Risk situationb | |
| Blood transfusion | 14 |
| Sex worker | 08 |
| HIV+ partner | 26 |
| Schooling | |
| ≤8 years | 231 (63.3) |
| >8 years | 123 (33.7) |
| Not informed | 11 (3.0) |
| Employment situation | |
| Employed | 69 (18.9) |
| Self-employed | 42 (11.5) |
| Unemployed | 61 (16.7) |
| Student | 25 (6.8) |
| Housewife | 117 (32.1) |
| Retired | 09 (2.5) |
| Not informed | 42 (11.5) |
When compared to the BED-CEIA, with a cutoff of 0.8, the Ax-AI showed 64.7% sensitivity of and 95.8% specificity, with rates of positive predictive values (PPV) and negative (NVP) greater than 85%. The agreement beyond chance between the two techniques was good (kappa=0.654, p<0.001).
BED-CEIA and Ax-AI results for six patients whose seroconversion date was equal to or less than a year, confirmed by a negative assay for HIV followed by a positive assay within 12 months, demonstrated disagreement in only two patients the classification status of recent infection. In one of these cases seroconverted after seven months and BED-CEIA ODn was borderline (at the cutoff threshold) (data not shown).
Global HIV-1 incidences according to BED-CEIA and Ax-AI were 0.79 (95% CI: 0.60–0.98) and 0.34 (95% CI: 0–0.72), respectively. Thus, the incidence rate estimated by the BED-CEIA was significantly higher (p>0.0001). The number of recent infections among HIV positive individuals, for the BED-CEIA, Ax-AI and BED+Ax-AI were 105, 77 and 66 (p=0.004). Only the Ax-AI demonstrated a significant increasing tendency of HIV incidence with time (p=0.02). The incidence estimates varied between 0.66 and 0.97%/year and 0.19 and 0.45%/year for the BED-CEIA and Ax-AI, respectively. All methods for estimating HIV-1 incidence showed significantly higher rates for men than for women (p<0.0001), with a difference of approximately threefold (Table 2).
Annual HIV-1 incidence estimated by using the BED-CEIA, Ax-AI, and BED-CEIA+Ax-AI algorithms.
| Year/gender | No. of individuals | No. of individuals | Incidence BED (%) – (95% CI)d | Incidence Ax-AI (%) – (95% CI)d | |||
|---|---|---|---|---|---|---|---|
| HIV positivea (BED samples)b (Ax-AI samples)c | HIV negative | Recent infection (BED) | Recent infection (Ax-AI) | Recent infection (BED+Ax-AI) | |||
| 2006 | 111a (66)b (64)c | 8931 | 18 | 11 | 09 | 0.70 (0.38–1.02) | 0.19 (0–0.63) |
| 2007 | 141 (98) (90) | 9242 | 29 | 20 | 16 | 0.97 (0.60–1.35) | 0.45 (0–0.94) |
| 2008 | 122 (118) (118) | 9208 | 34 | 26 | 24 | 0.81 (0.47–1.14) | 0.38 (0–0.81) |
| 2009 | 123 (93) (93) | 9998 | 24 | 20 | 17 | 0.66 (0.36–0.95) | 0.34 (0–0.74) |
| Male | 243 (180) (177) | 8449 | 44 | 37 | 32 | 1.46 (0.94–1.97) | 0.77 (0–1.65) |
| Female | 254 (195) (188) | 28,930 | 61 | 40 | 34 | 0.59 (0.42–0.76) | 0.24 (0–0.51) |
| Total | 497 (375) (365) | 37,379 | 105 | 77 | 66 | 0.79 (0.60–0.98) | 0.34 (0–0.72) |
A total of 10 HIV-positive patients were excluded from the incidence calculation: eight had presented a previous positive serological test for HIV, one was vertical transmission, and another was undergoing antiretroviral treatment. Three of these individuals were from the 2006 cohort, two were from the 2007 cohort, three were from the 2008 cohort, and two from the 2009 cohort.
The present study demonstrates that estimates of HIV incidence in a population seeking VCTs in two municipalities within the Metropolitan Region of Recife, capital city of Pernambuco, Northeast Brazil, may vary according to the diagnostic algorithm used, with a rate of 0.79%/year when using the BED and 0.34%/year with the Ax-AI, excluding those on HAART. The BED-CEIA methodology has been widely used to measure the incidence of HIV-1 in cross-sectional studies, mainly due to its commercial availability. However, many questions remain regarding its accuracy, with a suggestion of overestimation, despite the consensual use of correction factors.2 The WHO Working Group on HIV Incidence Assays systematically reviewed the accuracy of serological tests for recent infections with HIV. Across 13 different assays, sensitivity to detect recent infections ranged from 42 to 100% (median 89%). Specificity for detecting established infections was between 49.5 and 100% (median 86.8%) and was higher for infections of durations longer than one year (median 98%, range 31.5–100.0). Serological assays have reasonable sensitivity for detecting recent HIV infection, but are vulnerable to misclassifying established infections as recent, potentially leading to biases in incidence estimates.15 The use of a multi-assay algorithm has been suggested, in which BED-CEIA in combination with an avidity assay was cited as the most commonly used algorithm.2
The present study tested this strategy in individuals in the metropolitan area of Recife, Northeast Brazil, where the most prevalent subtypes B are (around 57%), F (around 37.7%) and C (around 3.1%).16 A significant difference was observed between incidence rates estimated by BED-CEIA (0.79%/year) and Ax-AI (0.34%/year) (p<0.00001).
One major difficulty when discussing estimates of HIV incidence is the difference among populations studied. Most studies have a target population of groups at risk, especially sex workers, men who have sex with men (MSM) and injected drug users. This research evaluated a mixed population from two VCTs in the Brazilian state of Pernambuco, which were linked to the Brazilian Ministry of Health's National STD and AIDS Programme. In this region, those attending the VCTs consist of three main groups: people from the general community, usually of low risk infection, pregnant women from within the community, and individuals with behaviour patterns associated with an increased risk of infection (e.g. sex workers and MSM). Thus, one limitation of this study among other studies in Brazil is the heterogeneity of the population studied plus the fact that the accuracy of the estimates of incidence by serological methods depends on the accuracy of the mean RITA duration and the RITA FRR, which are the measures evaluated for populations with characteristics different from those of the present study.17
There are data illustrating that the avidity test presents a better performance in correctly classifying individuals with a long-term viral load, while the BED-CEIA has greater tendency to classify them erroneously as recent infections.18 Furthermore, it has been shown that AI-Ax suffers less interference from the HIV subtype and antibody titres in their performance.6
The incidence estimates presented herein were about threefold higher for men than for women. The Brazilian epidemic is concentrated within certain groups, especially young MSM.1 However in the present study, this group accounted for only 14%, thus, underrepresented in the population of VCTs studied. In Brazil, there have been several studies, which applied serological methods to estimate HIV-1 incidence, especially in the South and Southeast. Although a considerable portion of these studies included individuals with a higher risk for HIV infection, such as prisoners, cocaine and injected drugs users, MSM and high-risk heterosexual men and women, incidence data for both sexes show a higher rate for men, as observed by Schechter et al.19 and Alves et al.,20 where the estimated incidence rate was between 1.9 and 2% for women and 2.8 and 2.7% for men and, respectively.
Given the large number of diagnostic tests performed for HIV infection (132 million/year) and blood bank screening (101 million/year), as well as recognizing the huge market involved, the importance of applying the RITA methodology should be emphasized in order to provide a better map of the spread of the epidemic and allow adequate structuring of preventive strategies. To our knowledge, this is the first study in Brazil to apply the concept of the dual testing algorithm to determine estimates of HIV incidence, based on the most recent recommendations of the WHO.2,14
FundingPrograma Nacional de Cooperação Acadêmica – Ação Novas Fronteiras (Procad – NF) – Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Ministério da Educação, Brasil.
Conflicts of interestThe authors declare no conflicts of interest.