Tuberculosis is an infectious disease of global importance with major economic and social burden accounting for 25% of all avoidable deaths in developing countries. Extrapulmonary involvement may occur either in association with clinically apparent pulmonary tuberculosis or in isolation. This cross-sectional descriptive study aimed to evaluate the impact of ocular tuberculosis in visual acuity at baseline and after two months of intensive anti-tuberculous therapy. A sample of 133 pulmonary tuberculosis patients, seven disseminated tuberculosis, and three pleural tuberculosis patients was evaluated. All patients underwent routine ophthalmic evaluation, including assessment of visual acuity, biomicroscopy, applanation tonometry, indirect ophthalmoscopy, and fluorescent angiography as appropriate. None of the patients had impaired visual acuity due to tuberculosis. A rate of 4.2% (6/143) of ocular involvement was found. None of the patients with ocular involvement were HIV-infected. Of the six patients with ocular involvement, five met the diagnostic criteria for probable and one for possible ocular lesions. As for the type of ocular lesions, two patients had bilateral findings: one had sclerouveitis and the second had choroidal nodules. The other four patients presented with unilateral lesions: peripheral retinal artery occlusion in the right eye (one case), choroidal nodules in the left eye (one case), and choroidal nodules in the right eye (two cases). Patients progressed favorably after two month of intensive therapy, with no significant reduction in vision.
Tuberculosis (TB) is an infectious disease of global importance with major economic and social burden, accounting for 25% of all avoidable deaths in developing countries.1 The main clinical form is pulmonary TB, but extrapulmonary disease occurs at varying frequencies in both immunocompetent and immunocompromised patients.2,3 Extrapulmonary involvement, including skin, kidney, central nervous system, and eyes may occur either in association with clinically apparent pulmonary TB or in isolation, with no clinical or bacteriological evidence of pulmonary infection. Ocular TB may result from hematogenous, primary exogenous, or direct contiguity dissemination. Hematogenous origin is the most common mode of infection. Ocular TB may involve ocular adnexa (orbit, eyelids, tear glands) as well as the eye globe.4,5
It can be especially difficult to identify. The current limitations of diagnostic criteria and lack of accurate information on eye disease could be attributed to several factors: (a) technical difficulties and risks of visual impairment involved in clinical specimen collection for definitive microbiological diagnosis; (b) inaccurate diagnostic criteria used in some studies, including presumptive diagnosis based on treatment response or strong tuberculin test reactors; (c) timing of ophthalmic examination and TB treatment (before or after starting treatment) as ocular lesions may regress or heal within weeks after anti-TB treatment initiation; and/or (d) the presence of comorbidities leading to an immunocompromised state, which undermines an effective inflammatory response and lesion development.6
The prevalence of ocular TB varies widely around the world. Few well-designed and controlled studies on ocular TB have been conducted, but still little is known about its impact on vision. Studies to date have only reported the frequency of ocular TB and its clinical manifestations without assessing visual acuity or have examined a single assessment of visual acuity without monitoring the effects of treatment.7–10 This study aimed to assess visual acuity of TB patients before and after two months of treatment with anti-tuberculous therapy.
Patients and methodsThis was a cross-sectional descriptive study approved by the institutional review board of the Universidade Federal do Espírito Santo (UFES). A total of 177 bacteriologically confirmed pulmonary and/or extrapulmonary TB patients were enrolled in the study. Patients were interviewed, examined and demographic, epidemiologic and clinical information was recorded in a standardized questionnaire. Informed consent was obtained from all participants included in the study. At the hospital's Ophthalmology clinic, two ophthalmologists examined all patients. Newly diagnosed and bacteriologically confirmed TB patients of any sex, age, or HIV status were included in the study. Patients with a prior history of TB, old chorioretinitis lesions observed during fundoscopic eye examination, or self-reported treatment with anti-tuberculous medications during the previous six months were excluded.
All 177 patients underwent the following ophthalmic evaluation: (1) inspection and examination of ocular adnexa and orbit; (2) visual acuity for each eye using a Snellen chart; (3) biomicroscopy with a slip lamp (Carl Zeiss SL 120 Biomicroscopic Slip amp) for examination of the anterior chamber of the eye; (4) tonometry with a tonometer (Goldmann Tonometer, Carl Zeiss AT 020); (5) binocular indirect ophthalmoscopy with a HEINE Binocular Indirect Ophthalmoscope (after mydriasis with 1% tropicamide eye drops). In addition to the tests described above, one patient also underwent fluorescent angiography (AFG) due to the presence of retinal vasculitis.
Diagnosis of ocular tuberculosisThe diagnosis of ocular TB was made based on the criteria for “probable” and “possible” disease according to Bousa, Merino, Sanchez-Munoz and Carrillo classification (1997). This diagnostic classification uses a three-level probability scheme: (1) definitive diagnosis – isolation of Mycobacterium tuberculosis from eye specimens; (2) probable diagnosis – isolation of M. tuberculosis from extraocular tissues or fluids when there are ocular lesions consistent with TB infection in one or both eyes (which cannot be attributed to other causes and there is adequate clinical response to anti-TB treatment); (3) possible diagnosis – same criteria as “probable” diagnosis, but with the inability to evaluate treatment response due lack of clinical follow-up of the patient. Intraocular biopsy specimens were not obtained as the patients enrolled in the study had been previously diagnosed with TB. As such, no definitive diagnosis was made in our sample; only “probable” and “possible” levels were used for the diagnosis of ocular TB.
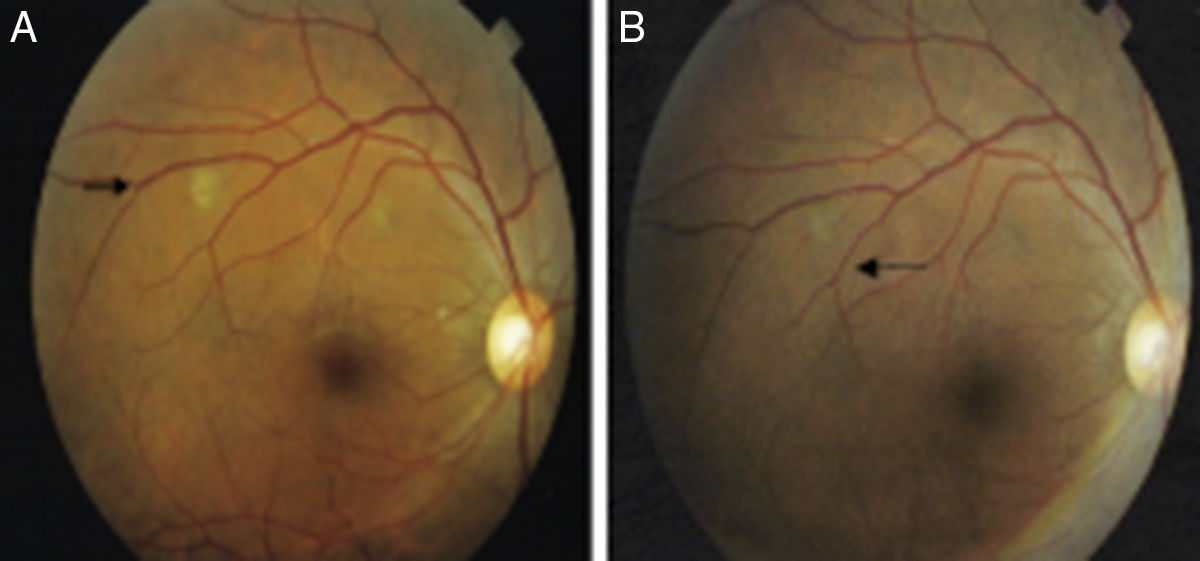
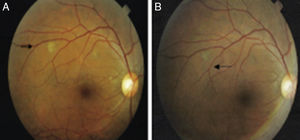
All ocular lesions consistent with TB were photographed, and the patients were reassessed within 60 days. At that time, new photographs were taken so that pre- and post-treatment photos could be compared and response to TB treatment could be ascertained (Fig. 1). An HIV test was offered to all patients during the study period. Those patients with ocular lesions in the anterior chamber were also tested for syphilis using treponemal and nontreponemal serologic tests.
Patient treatmentThe study treatment regimens for pulmonary TB consisted of two months of daily isoniazid (H), rifampin (R), pyrazinamide (Z), and ethambutol (E), followed by four months of daily HR (6-month standard short-course chemotherapy).11 At least five of the seven weekly doses of anti-tuberculosis treatment were administered by directly observed therapy. Patients with ocular TB followed official Brazilian guidelines for treating tuberculosis,19 which recommend that all forms of extrapulmonary TB (except meningitis) should be treated for six months, as well as patients coinfected with HIV. The primary ophthalmologic outcome was evaluated after two-months of anti-TB treatment based on previous studies that suggested a favorable therapeutic response by the end of intensive treatment phase.5,7
Statistical analysisThe sample consisted of 143 patients which equates to a sampling error of 3.4 of an estimated of population 1500 new cases of tuberculosis in a year with an expected prevalence of 5% and a significance level of 5%.
The associations between the presence of tuberculous lesions in the eye with vision or eye pressure were assessed using the Mann–Whitney U test. To compare the presence of tuberculous lesions in the eye with the eye examinations of prior and superior to such segments and the Chi-square test was required.
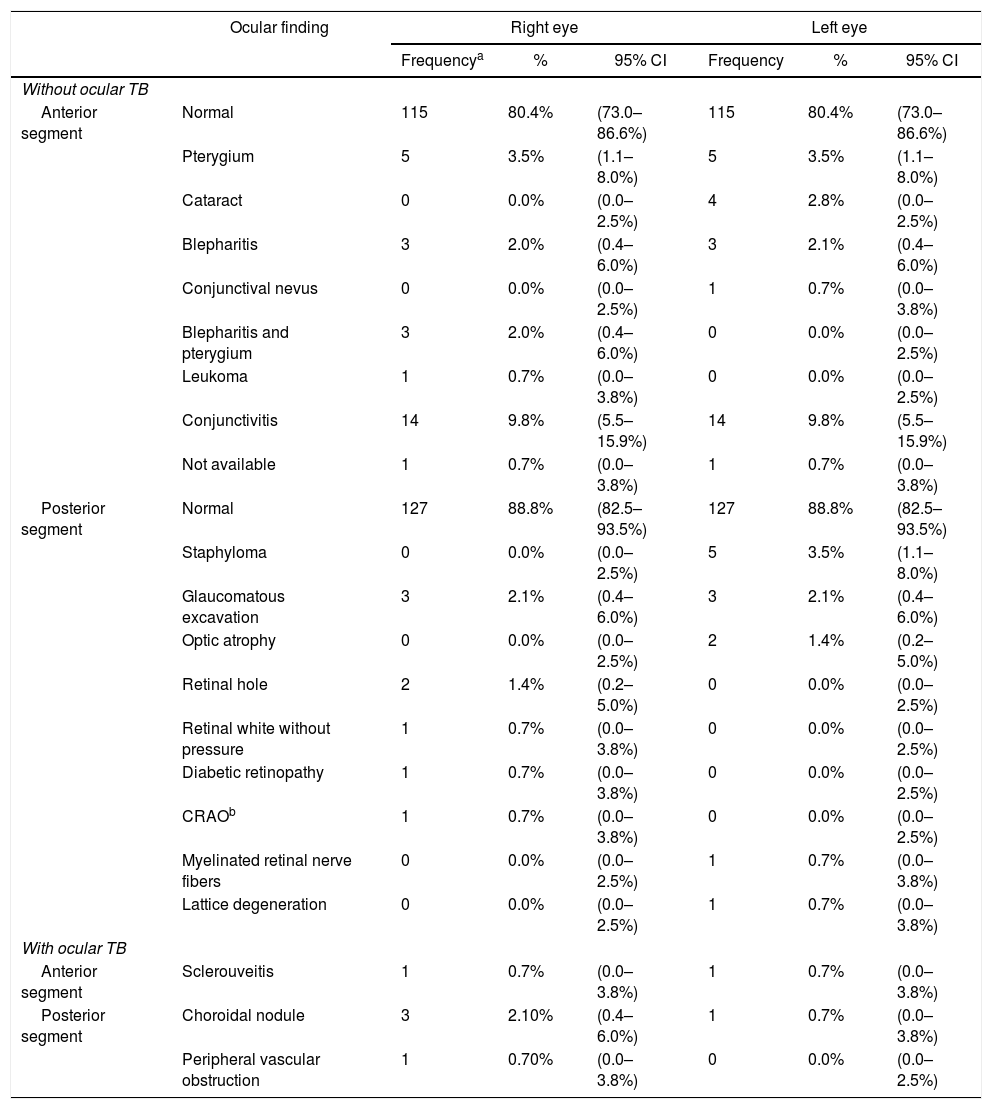
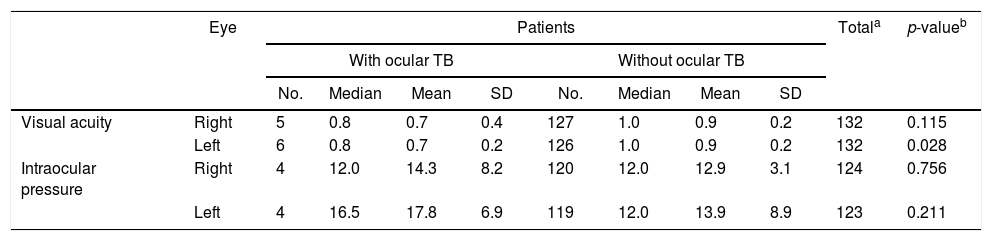
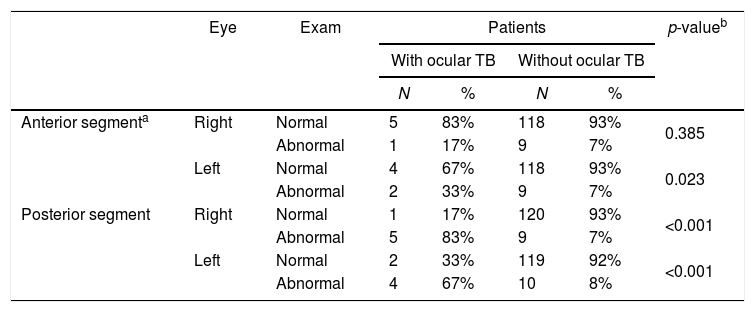
ResultsOne hundred and seventy-seven adults with suspected pulmonary TB were evaluated for study participation. Thirty-four patients were excluded because they did not meet the diagnostic criteria for tuberculosis. Of the 143 TB cases enrolled in the study, 133 (93.7%) had newly diagnosed initial episodes of pulmonary TB (culture positive), seven (4.9%) had disseminated TB, and three (1.4%) had pleural TB. Patients’ ages ranged from 18 to 79 years with mean age of 36.6±11.8 years. The male-to-female ratio was 2.7–1. Only seven individuals (4.9%) were inpatients at the time of enrollment, the remainder were evaluated on an outpatient basis. Six patients (4.2%) were co-infected with HIV, 131 (91.6%) tested negative for HIV, and six (4.2%) had no information available on their HIV status. Presence of cavities on chest radiograph was observed in most of the patients (>70%). Tuberculin skin test (TST) was performed in all patients enrolled and a strong reaction was seen in three patients with ocular TB. Of all patients included in the study, 93 (65%) had not started anti-TB treatment at the time of ophthalmic evaluation, and 43 (30%) patients were under treatment for less than seven days. Ocular TB was diagnosed in 4.2% of the patients (6/142). Table 1 shows ocular findings from both patients with and without ocular TB. Fundoscopic examination in patients without ocular TB revealed that most (>80%) had normal fundi, retinal vessels, optic nerves, and peripheral retinal vessels. The main findings in the anterior segment of patients without ocular TB were conjunctivitis (9.8%), followed by pterygium, and blepharitis, while in the posterior segment were staphyloma and glaucomatous excavation. Most tuberculosis patients (97.2%) showed good visual acuity. Results ranged from 0.0 to 1.0 (mean 0.85±0.2) and 0.1 to 1.0 (mean 0.86±0.2) in the right and left eyes, respectively. There was no statistically significant difference (p>0.05) in visual acuity in patients with ocular pressure and without occular TB (Table 2). However, dilated fundus and anterior segment examination revealed higher frequency of abnormal findings (p<0.05) in posterior segment of eye from patients with ocular TB (Table 3).
Ocular findings in newly diagnosed patients with tuberculosis.
| Ocular finding | Right eye | Left eye | |||||
|---|---|---|---|---|---|---|---|
| Frequencya | % | 95% CI | Frequency | % | 95% CI | ||
| Without ocular TB | |||||||
| Anterior segment | Normal | 115 | 80.4% | (73.0–86.6%) | 115 | 80.4% | (73.0–86.6%) |
| Pterygium | 5 | 3.5% | (1.1–8.0%) | 5 | 3.5% | (1.1–8.0%) | |
| Cataract | 0 | 0.0% | (0.0–2.5%) | 4 | 2.8% | (0.0–2.5%) | |
| Blepharitis | 3 | 2.0% | (0.4–6.0%) | 3 | 2.1% | (0.4–6.0%) | |
| Conjunctival nevus | 0 | 0.0% | (0.0–2.5%) | 1 | 0.7% | (0.0–3.8%) | |
| Blepharitis and pterygium | 3 | 2.0% | (0.4–6.0%) | 0 | 0.0% | (0.0–2.5%) | |
| Leukoma | 1 | 0.7% | (0.0–3.8%) | 0 | 0.0% | (0.0–2.5%) | |
| Conjunctivitis | 14 | 9.8% | (5.5–15.9%) | 14 | 9.8% | (5.5–15.9%) | |
| Not available | 1 | 0.7% | (0.0–3.8%) | 1 | 0.7% | (0.0–3.8%) | |
| Posterior segment | Normal | 127 | 88.8% | (82.5–93.5%) | 127 | 88.8% | (82.5–93.5%) |
| Staphyloma | 0 | 0.0% | (0.0–2.5%) | 5 | 3.5% | (1.1–8.0%) | |
| Glaucomatous excavation | 3 | 2.1% | (0.4–6.0%) | 3 | 2.1% | (0.4–6.0%) | |
| Optic atrophy | 0 | 0.0% | (0.0–2.5%) | 2 | 1.4% | (0.2–5.0%) | |
| Retinal hole | 2 | 1.4% | (0.2–5.0%) | 0 | 0.0% | (0.0–2.5%) | |
| Retinal white without pressure | 1 | 0.7% | (0.0–3.8%) | 0 | 0.0% | (0.0–2.5%) | |
| Diabetic retinopathy | 1 | 0.7% | (0.0–3.8%) | 0 | 0.0% | (0.0–2.5%) | |
| CRAOb | 1 | 0.7% | (0.0–3.8%) | 0 | 0.0% | (0.0–2.5%) | |
| Myelinated retinal nerve fibers | 0 | 0.0% | (0.0–2.5%) | 1 | 0.7% | (0.0–3.8%) | |
| Lattice degeneration | 0 | 0.0% | (0.0–2.5%) | 1 | 0.7% | (0.0–3.8%) | |
| With ocular TB | |||||||
| Anterior segment | Sclerouveitis | 1 | 0.7% | (0.0–3.8%) | 1 | 0.7% | (0.0–3.8%) |
| Posterior segment | Choroidal nodule | 3 | 2.10% | (0.4–6.0%) | 1 | 0.7% | (0.0–3.8%) |
| Peripheral vascular obstruction | 1 | 0.70% | (0.0–3.8%) | 0 | 0.0% | (0.0–2.5%) | |
Visual acuity and intraocular pressure of newly diagnosed patients with pulmonary, disseminated and pleural tuberculosis.
| Eye | Patients | Totala | p-valueb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| With ocular TB | Without ocular TB | ||||||||||
| No. | Median | Mean | SD | No. | Median | Mean | SD | ||||
| Visual acuity | Right | 5 | 0.8 | 0.7 | 0.4 | 127 | 1.0 | 0.9 | 0.2 | 132 | 0.115 |
| Left | 6 | 0.8 | 0.7 | 0.2 | 126 | 1.0 | 0.9 | 0.2 | 132 | 0.028 | |
| Intraocular pressure | Right | 4 | 12.0 | 14.3 | 8.2 | 120 | 12.0 | 12.9 | 3.1 | 124 | 0.756 |
| Left | 4 | 16.5 | 17.8 | 6.9 | 119 | 12.0 | 13.9 | 8.9 | 123 | 0.211 | |
Dilated fundus and anterior segment examination of newly diagnosed patients with pulmonary, disseminated and pleural tuberculosis.
| Eye | Exam | Patients | p-valueb | ||||
|---|---|---|---|---|---|---|---|
| With ocular TB | Without ocular TB | ||||||
| N | % | N | % | ||||
| Anterior segmenta | Right | Normal | 5 | 83% | 118 | 93% | 0.385 |
| Abnormal | 1 | 17% | 9 | 7% | |||
| Left | Normal | 4 | 67% | 118 | 93% | 0.023 | |
| Abnormal | 2 | 33% | 9 | 7% | |||
| Posterior segment | Right | Normal | 1 | 17% | 120 | 93% | <0.001 |
| Abnormal | 5 | 83% | 9 | 7% | |||
| Left | Normal | 2 | 33% | 119 | 92% | <0.001 | |
| Abnormal | 4 | 67% | 10 | 8% | |||
Of the six patients with ocular TB, none were co-infected with HIV. Three were inpatients at the time of ophthalmological examination and three were evaluated as outpatients. Out of these six patients three had miliary TB. The corrected visual acuity ranged from 0.1 to 1.0 (mean 0.7±0.3) and 0.5 to 1.0 (mean 0.9±0.2) on the right and left eye, respectively. Two had bilateral involvement: one patient had sclerouveitis and the second had multiple nodules in choroid. The other four patients had unilateral involvement: retinal vasculitis in the right eye (one case); choroidal nodules in the right eye (two cases); and choroidal nodules in the left eye (one case). Choroidal lesions were yellowish and ragged edged located at the posterior pole of the eye nodules. A good therapeutic response was observed in all patients with ocular TB. Regression of choroidal nodules (four patients) and retinal vasculitis (one patient) were evidenced after two month of TB treatment (Table 4). Corticosteroid therapy was not required in any patient since there were no serious lesions affecting optic disc and macula.
Therapeutic response after a two-month intensive anti-tuberculous treatment phase.
| Patient | Ag | Eye | VAa | Days on treatment: 0 (Baseline) | Eye | VAa | Days on treatment: 2 month | ||
|---|---|---|---|---|---|---|---|---|---|
| Ocular finding | Ocular finding | ||||||||
| Anterior segment | Posterior segment | Anterior segment | Posterior segment | ||||||
| 1 | 28/F | Right | 0.9 | Normal | Choroidal nodules | Right | 0.9 | Normal | Choroidal nodules regression |
| Left | 0.9 | Normal | Choroidal nodule | Left | 0.9 | Normal | Choroidal nodules regression | ||
| 2 | 62/M | Right | 0.8 | Pterygium | Choroidal nodules | Right | 0.8 | Pterygium | Choroidal nodules regression |
| Left | 0.6 | Pterygium | Normal | Left | 0.6 | Pterygium | Normal | ||
| 3 | 79/F | Right | 1.0 | Sclerouveitis | Drusen and optical nerve excavation | Right | 1.0 | Cured | Drusen and optical nerve excavation |
| Left | 1.0 | Sclerouveitis | Drusen | Left | 1.0 | Cured | Drusen | ||
| 4 | 34/M | Right | 1.0 | Normal | Normal | Right | 1.0 | Normal | Normal |
| Left | 1.0 | Normal | Choroidal nodule | Left | 1.0 | Normal | Choroidal nodules regression | ||
| 5 | 22/M | Right | 1.0 | Normal | Choroidal nodule | Right | 1.0 | Normal | Choroidal nodules regression |
| Left | 1.0 | Normal | Normal | Left | 1.0 | Normal | Normal | ||
| 6 | 47/M | Right | 1.0 | Normal | Drusen and optical disk excavation, retinal vasculitis. | Right | 1.0 | Normal | Drusen, optical disk excavation and retinal vasculitis regression |
| Left | 0.5 | Normal | Optical disk excavation | Left | 0.5 | Normal | Optical disk excavation | ||
Ocular TB can involve any part of the eye and can occur with or without evidence of systemic TB. Among patients with systemic tuberculosis, rates of ocular involvement have varied. A 1967 study of 10,524 patients in a Boston sanatorium, for example, reported a rate of ocular involvement of 1.4%. In contrast, a study in 1997 in 100 hospitalized patients with tuberculosis in Spain reported a rate of ocular involvement of 18%.5 Recently, Lara and Ocampo described 6.8% of ocular TB in 103 pulmonary TB patients evaluated in a tertiary hospital in the Philippines, and Mehta et al. reported 12.7% presumed ocular TB in 47 HIV/MDR-TB co-infected patients in Mumbai, India.12,13 The frequency of ocular TB found in our study (4.2%) lies within the mean range described in the literature.5,6,8–10
Aside from case reports, the only two Brazilian studies on ocular TB reported frequencies ranging between 0% and 5.5%.8,10 However, these studies have methodological biases that prevented a reliable comparison of frequencies. In one study the authors evaluated only non-HIV-infected inpatients at different time points of treatment, which may explain why no ocular lesions were found. The second study was affected by selection bias. Of the 16 cases classified as ocular TB, only 56% were bacteriologically confirmed in another organ, and six of 16 (38%) were HIV-infected.
After diagnosis of ocular TB, any of the three categories,5 it is important to assess vision acuity. All patients included in our study were suspected of having TB at the moment of ophthalmic evaluation and at that time there were no visual complaints, except when comorbidities were associated. Therefore, it is important to point out that even the six patients who were diagnosed with ocular TB in our study, only one had vision <0.6, probably associated with glaucoma. As we have evaluated patients with no vision complaints, it is possible that the extension of lesions was not severe enough to affect the visual acuity at that moment of ophthalmologic evaluation.
In a study conducted in Spain, visual acuity of 100 TB patients was assessed before and after treatment. The average visual acuity in patients with ocular involvement was lower (0.69±0.2) than in those with no ocular involvement (0.9±0.2). The authors described improved acuity in patients with healed lesions and argued that ocular TB affects vision.6 However, information on the exact timing of these assessments (at baseline and end of study) and treatment was not documented. In another study conducted in Burundi (Africa) in 154 AIDS patients, although 61% were co-infected with TB, ocular TB was not described. Visual acuity of 20/30 or more was reported in 80% of the eyes evaluated.9 In a study carried out in Brazil, Mendes et al. evaluated 292 patients with TB and found 5.5% with ocular involvement. The most common ocular lesions were choroidal tubercles (75%). Ophthalmic examinations were not timed with the treatment regimen and visual acuity was not monitored during treatment. However, the authors claimed that TB did not affect vision since only 18.75% of the patients had visual acuity better than 20/25.8
Out of the six patients with ocular TB in our study, three (50%) were hospitalized upon diagnosis (initial investigation) and had miliary TB. It is believed that miliary TB is a risk factor for ocular disease.14 In Brazil, Mendes et al. showed that seven (38%) of 18 patients with ocular TB had miliary disease (OR=8.13 95% CI 1.06–62.4).8 None of the six patients in our study had reduced visual acuity due to TB. In fact, five of the six patients had close to normal (≥0.8) visual acuity, and one patient had poor vision due to advanced glaucoma.
Choroidal nodules were found in four of the six patients (cases #1, 2, 4 and 5). One patient had bilateral sclerouveitis and one had temporal retinal vasculitis in the right eye. The highest number of nodules found per eye was two, although the literature has reported as many as 10–20 nodules per eye. Illingworth and Wright in 1948 were the first to describe these lesions. According to their report, choroidal nodules were single or multiple flat lesions measuring one to three-quarters of a disc.14–16 One of our cases had painful bilateral sclerouveitis that resolved with treatment showing significant segmental thinning and transparency of the underlying choroid (case #3). In 1941, Woods argued that TB was a primary cause of uveitis in 80% of cases. However, in his 1960 review, he recognized this rate was likely much lower – around 20%. In the 1990s, Weiner and Bezerra reported a 0.75% rate of uveitis. These inconsistent findings pointing toward lower rates may be explained by the development of new diagnostic technologies and methods, including laboratory techniques allowing for more accurate differential diagnoses of this form of ocular disease.16,17
Our study found only one case of retinal vasculitis (case #6). The first examination clearly showed areas of superficial and deep retinal hemorrhage and peripheral temporal vasculitis in the right eye. AFG showed extensive areas of retinal nonperfusion associated with contrast uptake in the walls of the affected vessels. After TB treatment the lesions regressed and laser therapy was not required as an adjunctive treatment.
In 2001, Gupta and colleagues evaluated 13 patients with PCR analysis positive for TB in aqueous and vitreous specimens. They found neovascularization in 11 eyes (57.8%), retinal hemorrhages in 10 (52.6%), preretinal or vitreous hemorrhages in five (26.3%) and these lesions regressed in all cases after a 12-month treatment. After choroidal nodules, periphlebitis is believed to be the second most important ocular manifestation of TB.18,19
In our study, all lesions were resolved in five of the six patients studied at the 60-day follow up. Two patients had lightly pigmented gray choroidal nodules suggestive of healing. One nodule was completely resolved with no scaring. The case of retinal vasculitis showed resolution of vascular inflammation and resorption of the hemorrhage. One case was lost to follow-up. All patients underwent ophthalmic examination within four days of specific treatment initiation and they all showed no signs of lesion resolution at that time point. Other authors have claimed that healing occurs around week eight of treatment. It is possible that treatment response occurs earlier than 60 days, but intermediate evaluations are necessary for a more accurate assessment.
In conclusion, TB-related ocular involvement (4.2%) included mostly choroidal nodules that progressed favorably after two months of intensive anti-tuberculous therapy, without causing any significant reduction in visual acuity. Although we have observed no eye damage after specific treatment, an accurate early diagnosis of ocular TB may be critical for preventing complications, such as retinal neovascularization, that can cause impaired vision or blindness if untreated. Moreover, since eye examination is not routinely performed in all TB patients, it is likely that disseminated TB is underreported worldwide.
Financial supportThis work was supported by master's grant; partnership Petrobrás – Universidade Federal do Espírito Santo.
Conflicts of interestThe authors declare no conflicts of interest.
We would like to thank Thiago Machado de Oliveira and Daniel Schott Teixeira da Silva, both from Boston University School of Medicine, for their assistance in editing and translating this text.