Zika virus (ZIKV) infection during pregnancy is associated with a congenital syndrome. Although the virus can be detected in human placental tissue and sexual transmission has been verified, it is not clear how the virus reaches the fetus. Despite the emerging severity caused by ZIKV infection, no specific prophylactic and/or therapeutic treatment is available. The aim of the present study was to evaluate the effectiveness antiviral of nitazoxanide (NTZ) in two important congenital transmission targets: (i) a primary culture of human placental chorionic cells, and (ii) human cervical epithelial cells (C33-A) infected with Brazilian ZIKV strain. Initially, NTZ activity was screened in ZIKV infected Vero cells under different treatment regimens with non-toxic drug concentrations for 48 h. Antiviral effect was found only when the treatment was carried out after the viral inoculum. A strong effect against the dengue virus serotype 2 (DENV-2) was also observed suggesting the possibility of treating other Flaviviruses. Additionally, it was shown that the treatment did not reduce the production of infectious viruses in insect cells (C6/36) infected with ZIKV, indicating that the activity of this drug is also related to host factors. Importantly, we demonstrated that NTZ treatment in chorionic and cervical cells caused a reduction of infected cells in a dose-dependent manner and decreased viral loads in up to 2 logs. Pre-clinical in vitro testing evidenced excellent therapeutic response of infected chorionic and cervical cells and point to future NTZ activity investigation in ZIKV congenital transmission models with the perspective of possible repurposing of NTZ to treat Zika fever, especially in pregnant women.
The Flavivirus genus comprises many viruses distributed worldwide which represent a serious risk to public health, such as the Zika virus (ZIKV) and the dengue virus (DENV).1 It is important to note, however, that no specific prophylactic and/or therapeutic treatment for both infections is yet available. However, special attention should be given to ZIKV infection, as it can represent a serious health concern during pregnancy regarding fetal development.2 ZIKV was first isolated in 1947 from a sentinel rhesus monkey in the Zika Forest in Uganda3 and currently circulates in Africa, Asia, Pacific and the Americas.4 The primary ZIKV transmission route to humans occurs through the bite of female Aedes hematophagous mosquitoes, especially Aedes aegypti.5
Both congenital and sexual ZIKV transmission have been reported, and viral particles in amniotic fluid, blood, urine, semen, saliva, cerebral and spinal cord fluids have been detected.6 ZIKV is the only arbovirus currently known to be sexually transmitted, and its RNA was detected in semen for long periods of time.7,8 ZIKV infection usually causes mild and self-limiting disease, but some cases progress to Guillain-Barré syndrome.9 However, a Zika outbreak in Brazil between 2015–2016 was associated with microcephaly and other neurological disorders in fetuses/newborns born to infected pregnant women,10 which was further defined as Congenital Zika Syndrome (CZS). The mechanisms by which the virus reaches the fetus are unclear. It is possible that the virus crosses the placenta through host cell lesions or infection.11,12 Several reports have indicated that ZIKV infects different placenta cells throughout pregnancy.11,12
The antiviral efficacy of several compounds has been tested in both in vitro and in vivo models, but few are in clinical trial.13 Some of these compounds are currently being tested in phases I or II clinical trials. For example, the BCX4430 analog, a nucleoside, was effective in preclinical testing against ZIKV PRVABC-59, MR-766 and P6-740 strains in AG129 mice and is undergoing Phase 1 clinical trials to assess safety, tolerability and pharmacokinetics.14 The Ebselen antioxidant is in phase II clinical trials, reported as effective against PRVABC-59 strain in AG129 and C57BL/6 mice.15
In the context of drug repurposing, antiviral evaluations of drugs approved by the Food and Drug Administration (FDA) have been conducted with the expectation of accelerating the discovery of an anti-ZIKV drug.16 For example, chloroquine, a clinical drug used for malaria treatment, has shown antiviral activity against ZIKV,17 sofosbuvir, used to treat hepatitis C, demonstrated activity in vitro and in vivo models against ZIKV,18 yellow fever19 and Chikungunya infection.20 Ribavirin, also used to treat hepatitis C, displays potent in vitro anti-ZIKV activity, and effectively suppressed viremia in ZIKV-infected STAT1-deficient mice,21 although its clinical use is contraindicated in pregnant women. Furthermore, nitazoxanide a thiazolide class drug used to treat viral gastroenteritis, helminthiasis, amebiasis, giardiasis, cryptosporidiosis, balantidiasis and isosporiasis has recently been reported as exhibiting activity against Zika virus in human alveolar adenocarcinoma epithelial cell lines, Vero cells, human microvascular endothelial cells and Human Neural Progenitor Cells.22,23 However, no clinical trial data on the effectiveness of these drugs against ZIKV are available.
Nitazoxanide (NTZ), an FDA-approved drug safe for pediatric clinical use and for pregnant women when the benefit justify the risk to the fetus, has shown potent activity against several microorganisms and in reducing Zika virus infection. Herein the antiviral effect of NTZ was assessed on two important human cells regarding pathophysiology of congenital ZIKV transmission. The results indicate that NTZ is highly effective against ZIKA virus in both a primary culture of chorionic human placental cells and in cervical cell lineage, with chorionic cells, in particular, displaying an excellent therapeutic window. These results support the expectation that the effectiveness of NTZ may prevent the virus from reaching the fetus in tests using congenital in vivo infection model.
Materials and methodsCell culturesChorionic cells were obtained from placentas at term (38–40 weeks) of pregnant women with no TORCH group infections or genetic and clinical alterations, who received care at the National Institute of Women, Child and Adolescent Health Fernandes Figueira-FIOCRUZ (CAAE 88642218.1.0000.52-69). After placenta collection (n = 5), the amnionchorionic membrane was mechanically separated and the maternal face (chorionic cells) was washed in phosphate buffered saline (PBS) to remove blood clots, followed by an enzymatic dissociation protocol adapted from Kliman et al.24 Briefly, the membrane was chopped with a scalpel and subjected to enzymatic dissociation cycles with 0.25% trypsin and 100 µg/mL of a DNase solution (Gibco, Waltham, MA, USA), under agitation at 37 °C. The chorionic cell suspension was centrifuged and filtered through a 100 μm mesh, and the pellet was resuspended in Dulbecco's Modified Eagle Medium: Nutrient Mixture F-12 (DMEM F-12; Gibco, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Cultilab, Campinas, SP, BR), 2% l-glutamine (Gibco, Waltham,MA, USA) and 1% penicillin/streptomycin (Gibco, Waltham, MA, USA). The chorionic cells were plated in culture flasks and maintained at 37 °C under a 5% CO2 atmosphere. The human cervical cell line (C33-A) and Vero cell line (kidney epithelial cells from African green monkey) were maintained at 37 °C under a 5% CO2 atmosphere in RPMI 1640 Medium (Sigma-Aldrich, St. louis, MO, USA) supplemented with 10% FBS, 2% l-glutamine and 1% penicillin/streptomycin. The C6/36 cell line from Aedes albopictus mosquito was maintained at 28 °C in Leibovitz's l-15 Medium (L-15; Gibco, Waltham, MA, USA) supplemented with 10% FBS, 10% tryptose phosphate (Sigma-Aldrich, St. louis, MO, USA), 1% l-glutamine, 1% nonessential amino acids (Gibco, Waltham, MA, USA) and 1.5% sodium bicarbonate (Sigma-Aldrich, St. louis, MO, USA) and 1% penicillin and streptomycin.
VirusThe Zika virus BRZIKV_AB_ES strain was isolated from a patient's serum in Vero cells (GenBank Accession No. KX212103; Espírito Santo) and the dengue virus DENV-2 44/2 strain was isolated in C6/36 cells (56344; Vitória, Espírito Santo).25 The stocks of both viruses were grown on C6/36 cells through three passages and subsequently titrated in Vero cell cultures by plaque forming unit assay (3,3 × 109 PFU/mL of ZIKV and 4 × 108 of DENV-2 PFU/mL) and stored at –70 °C. All experiments were carried out with the same viruses’ stocks.
DrugsNitazoxanide (NTZ) is a drug available in many countries worldwide, approved by the FDA in the USA and ANVISA in Brazil (Fig. 1S). Powdered form of the drug (Annita®) was used to prepare stock solutions (20 mg/mL) in an 100% dimethyl sulfoxide solution (DMSO; Sigma-Aldrich, St. Louis, MO, USA) and stored at 4 °C. Fresh dilutions were prepared in culture medium immediately prior to the assays.
Cytotoxicity assayNon-infected cell cultures seeded in 96-well plates were treated with 2-fold serially diluted NTZ (ranging from 12.5 to 1600 µg/mL) or 0.5–2% DMSO (control) and maintained at 37 °C under a 5% CO2 atmosphere for 48 h. Subsequently, cellular viability was measured by incubation with 5 mg/mL methyl thiazolyl tetrazolium reagent (MTT; Sigma-Aldrich, St. louis, MO, USA) for 4 h at 37 °C. After this time, the formazan crystals formed from viable cell metabolism were dissolved by the addition of 10% DMSO. Absorbances were determined in a spectrophotometer at 570 nm, allowing for determination of the percentage of viable cells and cytotoxic concentration that reduces 50% of cell culture viability (CC50) though a nonlinear regression analysis (dose-response curve) using the GraphPad Prism 8.0 software (GraphPad Software Inc., San Diego, CA, USA).
Viral infectionsAccording to cell type, the cultures were infected with ZIKV at different multiplicities of infection (MOI) and maintained for 24–72 h at 37 °C under a 5% CO2 atmosphere. Previous data indicate that Vero cells are very susceptible to ZIKV infection. Thus, the cultures were infected at a MOI of 1 for screening treatments with NTZ. Regarding DENV-2 infection, Vero cell cultures are more resistant and were infected at a MOI of 10 to assess whether NTZ is effective against other Flaviviruses. C6/36 cells infected with ZIKV at a MOI 1 exhibited low levels of infection, so the cultures were infected at a MOI of 10 to assess antiviral efficacy of NTZ in non-mammalian cells. As previous data indicate that chorionic cells were highly resistant to ZIKV infection at MOI 1 and MOI 10, the cultures were infected at a MOI of 20 for antiviral evaluation. On the other hand, C33-A cells are moderately resistant and were infected at a MOI of 10 for antiviral evaluation. After two hours, all cultures were washed with PBS to remove non-internalized viruses and fresh RPMI or DMEM-F12 media supplemented with 2% FBS was added.
Antiviral activityInfected cell cultures were treated with 2-fold serially diluted non-toxic concentrations of NTZ (12.5 µg/mL to 100 µg/mL), defined through a cytotoxicity assay, or with 0.5% DMSO solution without NTZ (vehicle control) in two regimens: (i) 24 h before or (ii) two hours after viral inoculum. Regarding antiviral assays, the cultures were monitored during 24 and 48 h after infection to calculate the percentage of infected cells and determine the half-maximal effective concentration (EC50) through a nonlinear regression analysis (dose-response curve) using the GraphPad Prism 8.0 software (GraphPad Software Inc., San Diego, CA, USA). This analysis allows the calculation of the selective index (SI), a ratio that measures the window between cytotoxicity and antiviral effect (CC50/EC50). The viral load was measured in the culture supernatants and the plaque assay was performed to qualitatively analyze plaque-forming reduction upon treatment.
ImmunofluorescenceThe viral envelope protein (E) of Flaviviruses (ZIKV and DENV-2) was assessed in infected and NTZ treated cell cultures to evaluate the percentage of infected cells and antiviral activity. The cultures were fixed in 4% paraformaldehyde (Sigma-Aldrich, St. louis, MO, USA) for 20 min at 4 °C, washed three times with PBS, permeabilized with 0.1% Triton X-100 (Thermo Fisher Scientific, Waltham, MA, USA) for five minutes, washed once more and then saturated with 3% bovine serum albumin (BSA; Sigma-Aldrich, St. louis, MO, USA) for 30 min. Samples were incubated with 4G2 mouse antibody (LATAM/Biomanguinhos) for 1 h/37 °C and, after washing twice with PBS, incubated with AlexaFluor 488-conjugated secondary antibody (Thermo Fisher Scientific, Waltham, MA, USA) for 1 h/37 °C. For host cell nucleus visualization, the samples were incubated with 10 µg/mL 4, 6-diamidino-2-phenylindole (DAPI; Thermo Fisher Scientific, Waltham, MA, USA) and mounted with 2.5% 1.4-diazabicyclo- (2.2.2)- octane (DABCO; Sigma-Aldrich, St. louis, MO, USA) to prevent fading and analyzed under a Axio Observer Z1 Motorized Inverted Fluorescence Microscope (Carl Zeiss, Oberkochen, Germany). Images were processed using the ImageJ software (ImageJ Image Processing and Analysis in Java) and CellProfiler (CellProfilerTM cell image analysis software) software for infected cell quantification.
Plaque forming assayMonolayers of Vero cells in 24-well plates were exposed to 100 µL/well of the supernatant from infected and treated cultures for one hour at 37 °C to assess plaque forming by released infectious viral particles. Next, cells were washed with PBS and RPMI containing 1% FBS and 3% carboxymethylcellulose (CMC; Sigma-Aldrich, St. Louis, MO, USA) (overlay medium) was added. After seven days at 37 °C, the monolayers were fixed with 10% formaldehyde (Sigma-Aldrich, St. louis, MO, USA) in PBS and stained with a 0.04% crystal violet solution (Sigma-Aldrich, St. Louis, MO, USA), for qualitative evaluations regarding plaque-forming reduction.
Molecular detection of virus RNA levelsViral RNA was extracted from the supernatant of ZIKV-infected and NTZ-treated cultures using the High Pure Viral Nucleic Acid Extraction Commercial Kit (Roche Diagnostics Brazil Ltda., São Paulo, SP, BR) ZIKV cDNA quantification was performed by the PCR technique through AgPath-ID One Step RT-PCR Kit (Thermo Fisher Scientific, Waltham, MA, USA), using primers and probes (Table 1S) published previously by Corman and collaborators.26 A synthetic standard curve (Table 1S) was designed to detect and quantify viral load, with detection limit of 10² copies/mL, R2 = 0.989 and Slope = 3.453.
Statistical analysesThe statistical analysis was carried out using the GraphPad Prisma 8.0 software (GraphPad Software Inc., San Diego, CA, USA), with the following levels of significance: **p < 0.0013, ***p = 0.0009, and ****p < 0.0001. All analyses were performed using one-way analysis of variance ANOVA with Dunnett’s multiple comparisons test. The data are representative of 3–5 experiments run in duplicate.
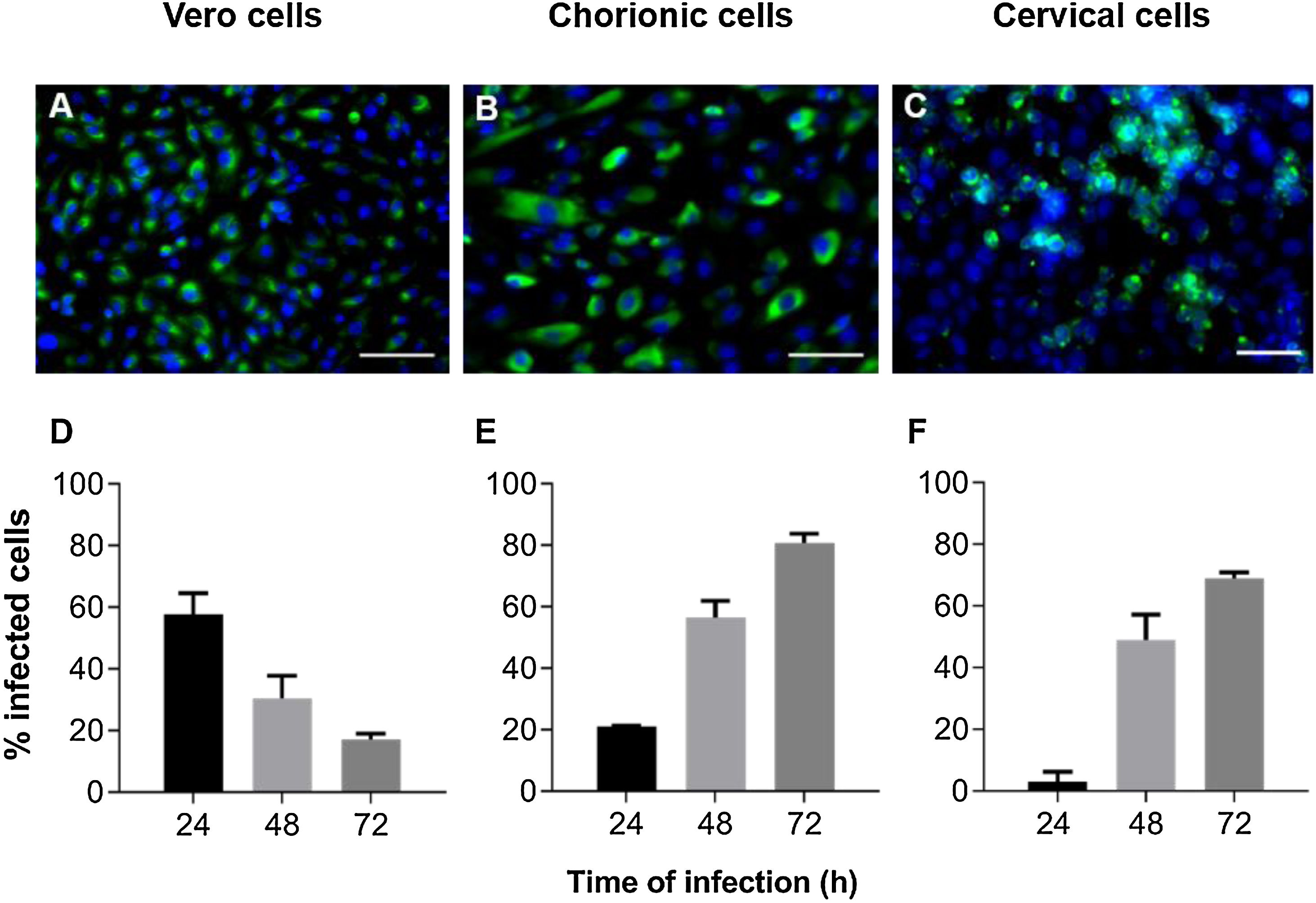
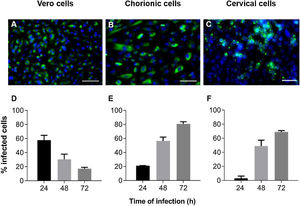
ResultsZIKV infection kinetics in Vero, chorionic and cervical cellsInfection kinetics were determined to assess the ZIKV infection profile of each cell type (Fig. 1). Vero cell cultures infected with MOI 1 exhibited decreased infection percentage in a time-dependent manner, with values of 58 ± 6.8, 30 ± 7.4 and 17 ± 1.9% at 24 h, 48 h and 72 h, respectively (Fig. 1D). Decreased infection was a consequence of cellular death resulting from cytopathic viral effect. On the other hand, chorionic cells showed increased values in a time-dependent manner of 21 ± 0.3% at 24 h, 57 ± 5.4% at 48 h and 81 ± 3.0% at 72 h of infection (Fig. 1E). The same infection profile was observed in cervical cell cultures, with increasing values from 3.1 ± 3.1 to 49 ± 8.1 and 69 ± 1.9 during kinetic infection (Fig. 1F). Intense labeling of the viral envelope protein (E) were observed in Vero cells infected at a MOI of 1 for 24 h (Fig. 1A), chorionic cells infected at a MOI of 20 (Fig. 1B) and cervical cells infected at an MOI of 10 (Fig. 1C) for 48 h, whose infection rates were similar, at about 58%, 57% and 49%, respectively. These experimental conditions were employed to evaluate treatment regimens in Vero cell cultures and antiviral activity in chorionic and cervical cells.
Kinetics of ZIKV infection in cell cultures. The detections of the viral antigen (green) and nucleus of host cells (blue) were performed by immunofluorescence: (A) Vero cells were infected with ZIKV at a MOI of 1 for 24 h, (B) chorionic cells at a MOI of 20 and (C) cervical cells at a MOI of 10 for 48 h. The graphs represent the means ± standard deviations of the percentage of infected cells during the infection kinetics in (D) Vero, (E) chorionic and (F) cervical cells. The data are representative of 3–5 experiments run in duplicate. Bars (A and B) 100 µm, (C) 50 µm.
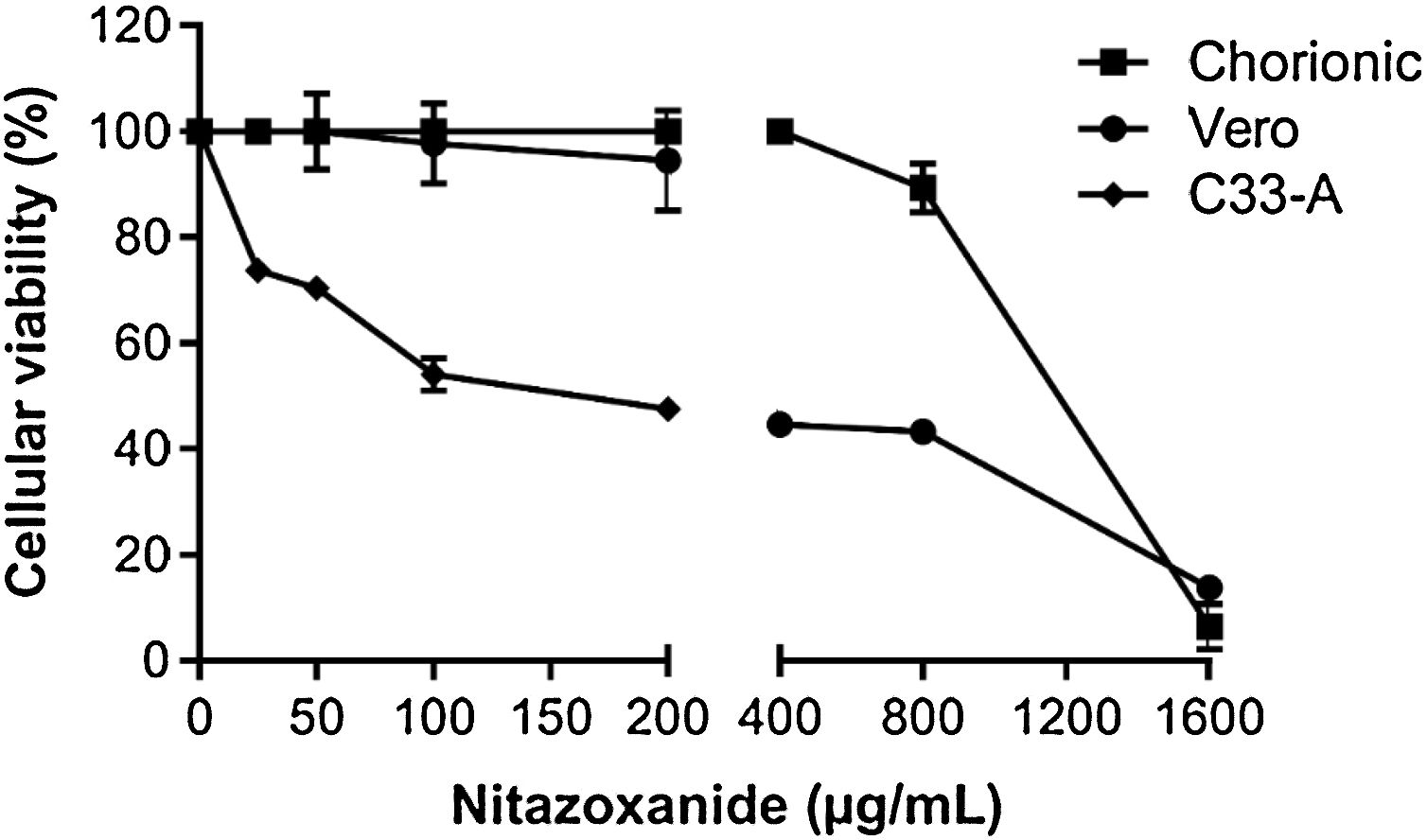
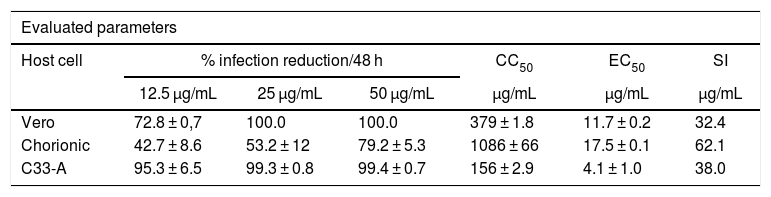
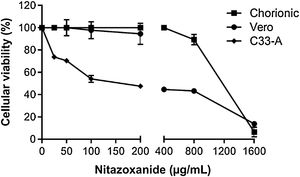
In order to rule out toxic effects of the drug on host cells, uninfected cells were incubated with different NTZ concentrations and cell viability was determined by the MTT assay (Fig. 2). After 48 h of treatment, C33-A cells (Fig. 2) and C6/36 (Fig. 2S E) were found to be more sensitive to NTZ toxicity than chorionic and Vero cells (Fig. 2). CC50 values for C6/36 and C33A cells were 134 ± 4.9 µg/mL and 156 ± 2.9 µg/mL, respectively (Fig. 2S E, Fig. 2, Table 1), while the CC50 value for Vero cells was of 379 ± 1.8 µg/mL and of 1086 ± 66 µg/mL for chorionic cells (Fig. 2, Table 1). Due to different levels of sensitivity to NTZ treatment observed between host cells, doses of 12.5 µg/mL to 50 µg/mL were selected for the antiviral assays for C6/36 and C33A cells and up to 100 µg/mL for Vero and chorionic cells, which maintained about 80% of cellular viability.
Cellular viability evaluated by the MTT assay. The C33-A cell line was treated with 0 to 200 μg/mL of the drug. The Vero cell line and the primary culture of chorionic cells were treated with 0 to 1600 μg/mL of the drug for 48 h. The graph represents the means ± standard deviations of the percentage of cellular viability. The data are representative of 3–5 experiments run in duplicate.
Antiviral activity of Nitazoxanide on host cells infected with ZIKV.
| Evaluated parameters | ||||||
|---|---|---|---|---|---|---|
| Host cell | % infection reduction/48 h | CC50 | EC50 | SI | ||
| 12.5 µg/mL | 25 µg/mL | 50 µg/mL | µg/mL | µg/mL | µg/mL | |
| Vero | 72.8 ± 0,7 | 100.0 | 100.0 | 379 ± 1.8 | 11.7 ± 0.2 | 32.4 |
| Chorionic | 42.7 ± 8.6 | 53.2 ± 12 | 79.2 ± 5.3 | 1086 ± 66 | 17.5 ± 0.1 | 62.1 |
| C33-A | 95.3 ± 6.5 | 99.3 ± 0.8 | 99.4 ± 0.7 | 156 ± 2.9 | 4.1 ± 1.0 | 38.0 |
CC50, Cytotoxic concentration 50% (µg/mL); EC50, Effective concentration 50% determined by percentage of the reduction of ZIKV infection (µg/mL)/48 h; SI, Selectivity Index (CC50/EC50).
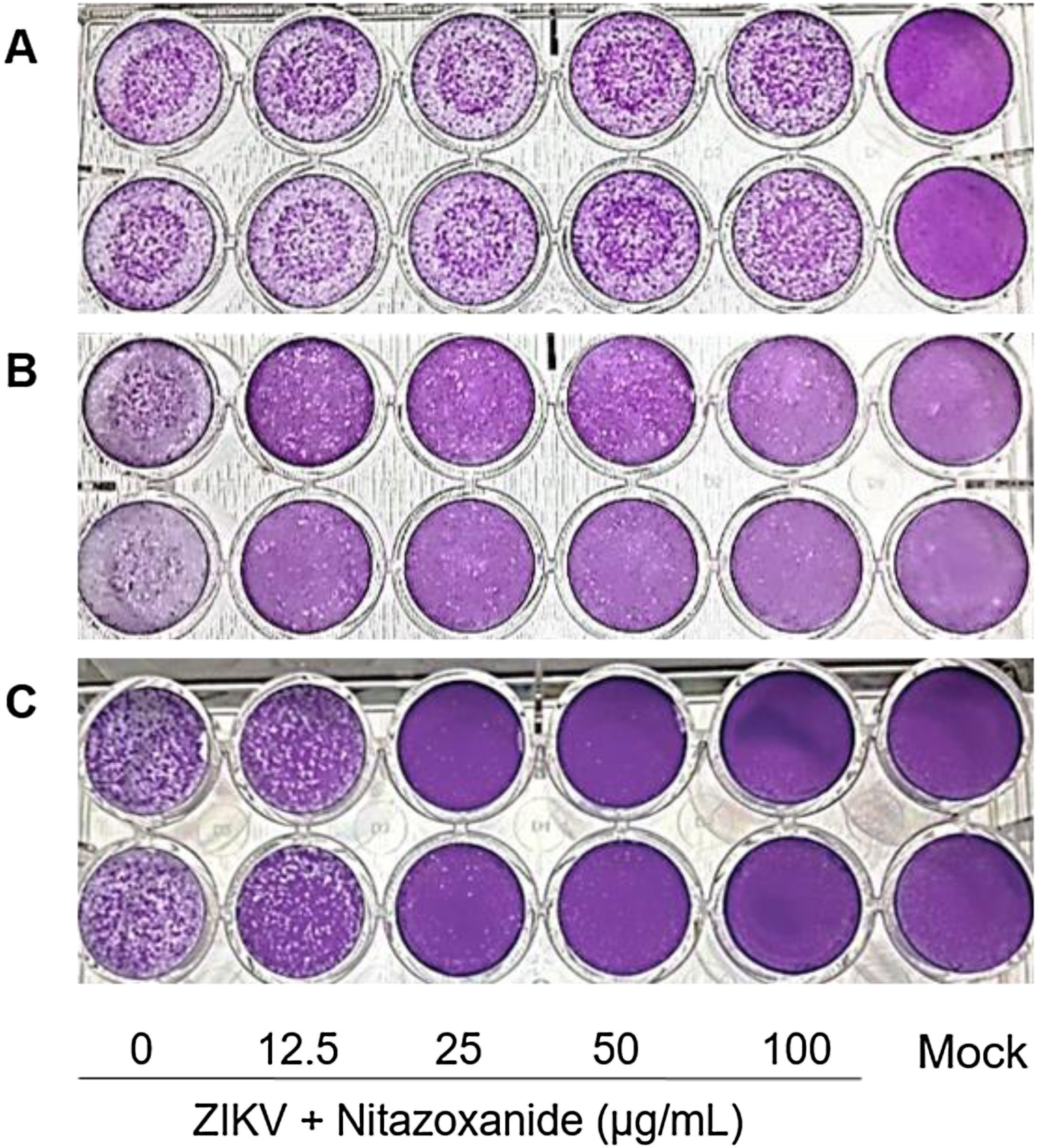
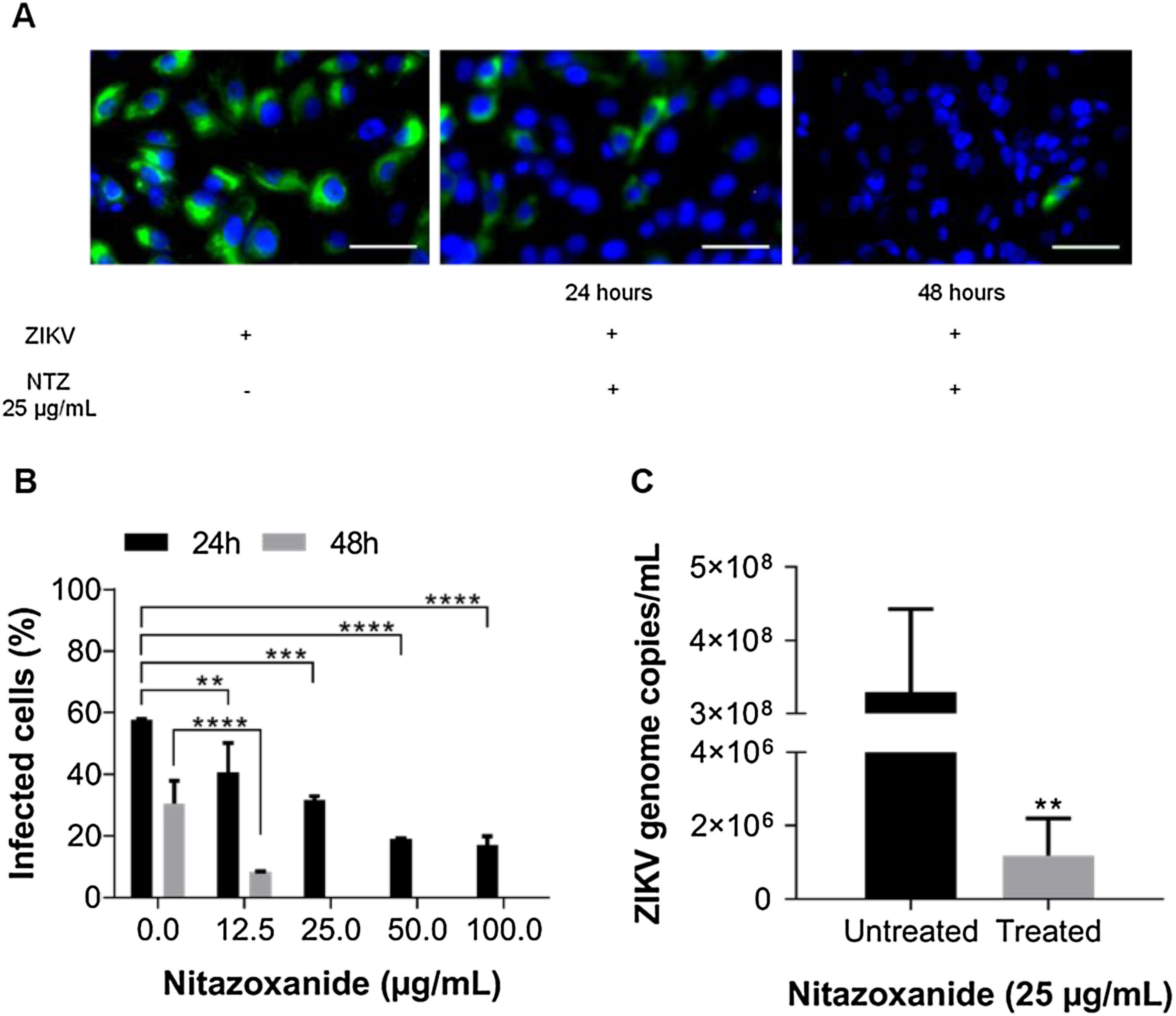
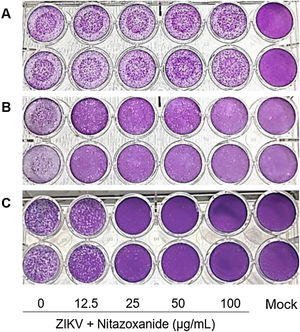
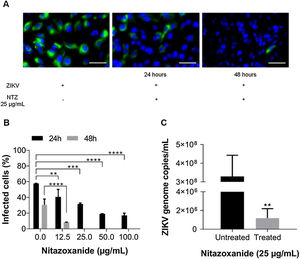
First, NTZ antiviral activity was screened in Vero cells by plaque forming (Fig. 3), immunofluorescence (Fig. 4A and B) and RT-qPCR assays (Fig. 4C). The treatment of infected cultures 24 h before the viral inoculum did not inhibit the production of infectious viruses (Fig. 3A). The same cytopathic effect was observed compared to untreated cultures (0 µg/mL), even at the highest tested concentration (100 µg/mL) (Fig. 3A). However, when treating after viral entry, the results showed a time and dose-dependent effect against ZIKV (Fig. 3B and C, Figs. 4A and B). Antiviral activity was stronger 48 h post-infection, where complete inhibition of infectious virus production was reached at 100 μg/mL NTZ, observed by the absence of plaque forming (Fig. 3C). Immunofluorescence applied to detect the intracellular viral antigen (E protein) indicated a time-dependent effect, with an important reduction in positively stained cells in the culture treated with 25 μg/mL NTZ compared to untreated cultures (Fig. 4A). The percentage of infected cells was significantly reduced by 66.9 ± 0.5% at 24 h post-infection when treated with 50 μg/mL NTZ, and by 72.8 ± 0.8% at 48 h post-infection when treated with 12.5 μg/mL NTZ, while at 25 μg/mL NTZ at 48 h post-infection 100% of infection was inhibited as compared to untreated cultures (Fig. 4B, Table 1). The IC50 value was 11.7 ± 0.2 μg/mL and the selective index (SI) was 32.4 after 48 h of infection (Table 1). When 100% of intracellular infection was eliminated at 25 μg/mL (Fig. 4B), RNA accumulation was quantified in the culture supernatants by RT-qPCR (Fig. 4C). The results indicate a greatly reduced viral load to 1.2 ± 1.0 × 106 copies/mL compared to 3.3 ± 1.1 × 108 copies/mL in untreated cultures (Fig. 4C), resulting in a significant 2-log amplitude reduction in viral load. Whereas efficacy of NTZ treatment in non-mammalian cells was not observed, C6/36 insect cells infected with ZIKV did not reduce the production of infectious viral particles after NTZ treatment (Fig. 2S F). On the other hand, NTZ activity against DENV-2 was observed in Vero cells (Fig. 3S), with a dose-dependent decrease in the percentage of infection (Fig. 3S C) and plaque formation (Fig. 3S D). Infection reductions at 50 μg/mL and 100 μg/mL NTZ of 96.4 ± 4.1% and 99.5 ± 0.7% were observed, respectively (Fig. 3S C). Taken together, these results indicate a strong effect of NTZ in inhibiting ZIKV and DENV-2 infection in Vero cells after viral entry, probably at the viral replication step.
Antiviral effect of Nitazoxanide determined by the plaque assay. Vero cell cultures were treated or not (Mock) with 12.5, 25, 50 and 100 μg/mL of the drug and infected with ZIKV at a MOI of 1. Treatment was performed (A) 24 h before infection and (B and C) after the viral inoculum. (A and B) the infection was followed-up until 24 h and (C) until 48 h. The data are representative of 3–5 experiments run in duplicate.
Antiviral effect of Nitazoxanide detected by immunofluorescence and RT-qPCR. Vero cell cultures were infected with ZIKV at a MOI of 1 and subsequently treated with 12.5, 25, 50 and 100 μg/mL of the drug for 24 h and 48 h. (A) The viral antigen (green) and the host cell nucleus (blue) detections were performed by immunofluorescence. (B) The graph represents the means ± standard deviations of the percentage of infected cells after 24 h and 48 h of treatment. (C) The supernatant from cultures treated with 25 μg/mL was collected 48 h after infection to quantify the viral load by the RT-qPCR assay. The graph represents the means ± standard deviations of the copies of the ZIKV genome/mL. Statistical significance was determined by a one-way ANOVA, followed by Dunnett’s multiple-comparisons test. **p < 0.0013, ***p = 0.0009, ****p < 0.0001. The data are representative of 3-5 experiments run in duplicate. Bars 50 µm.
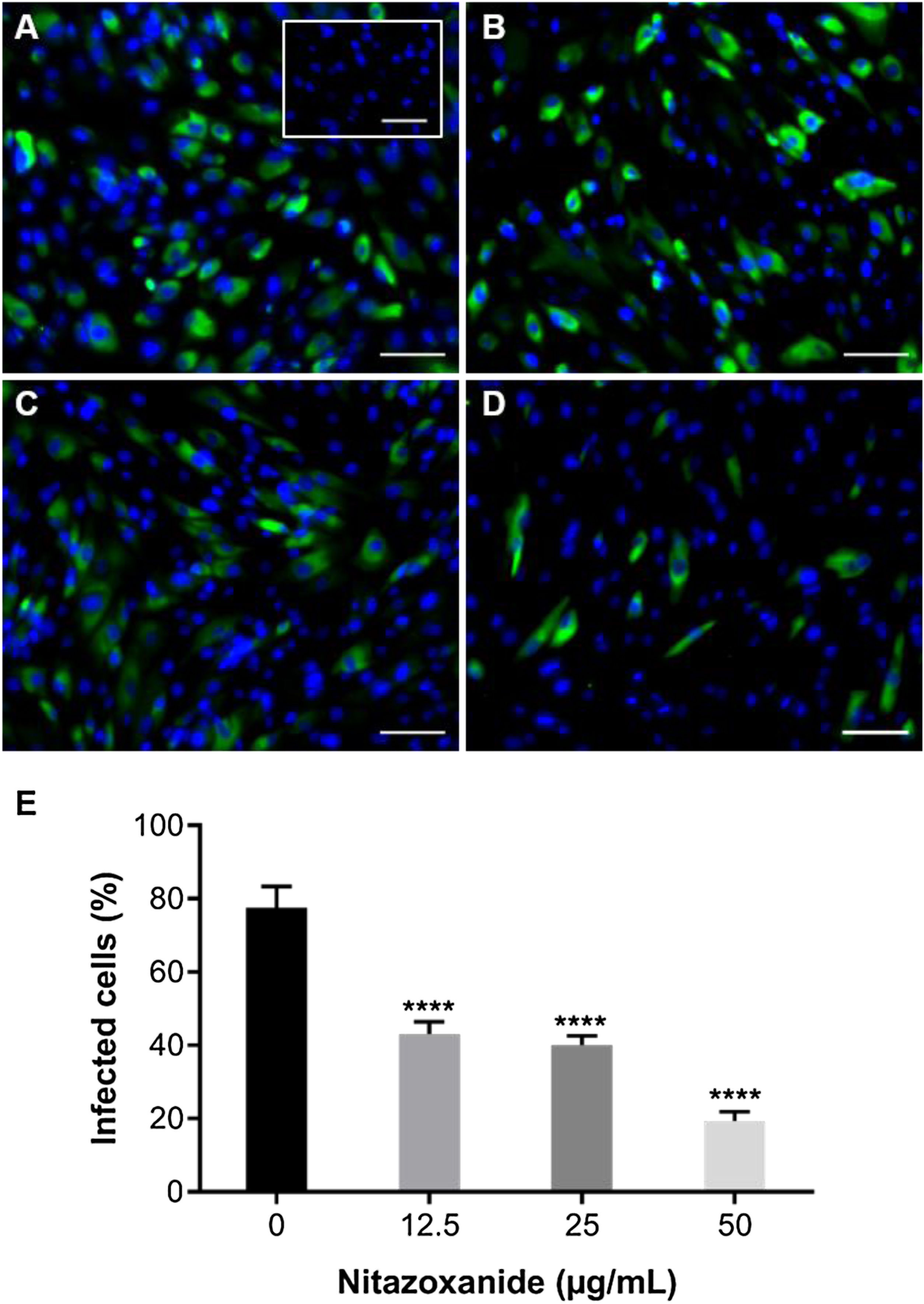
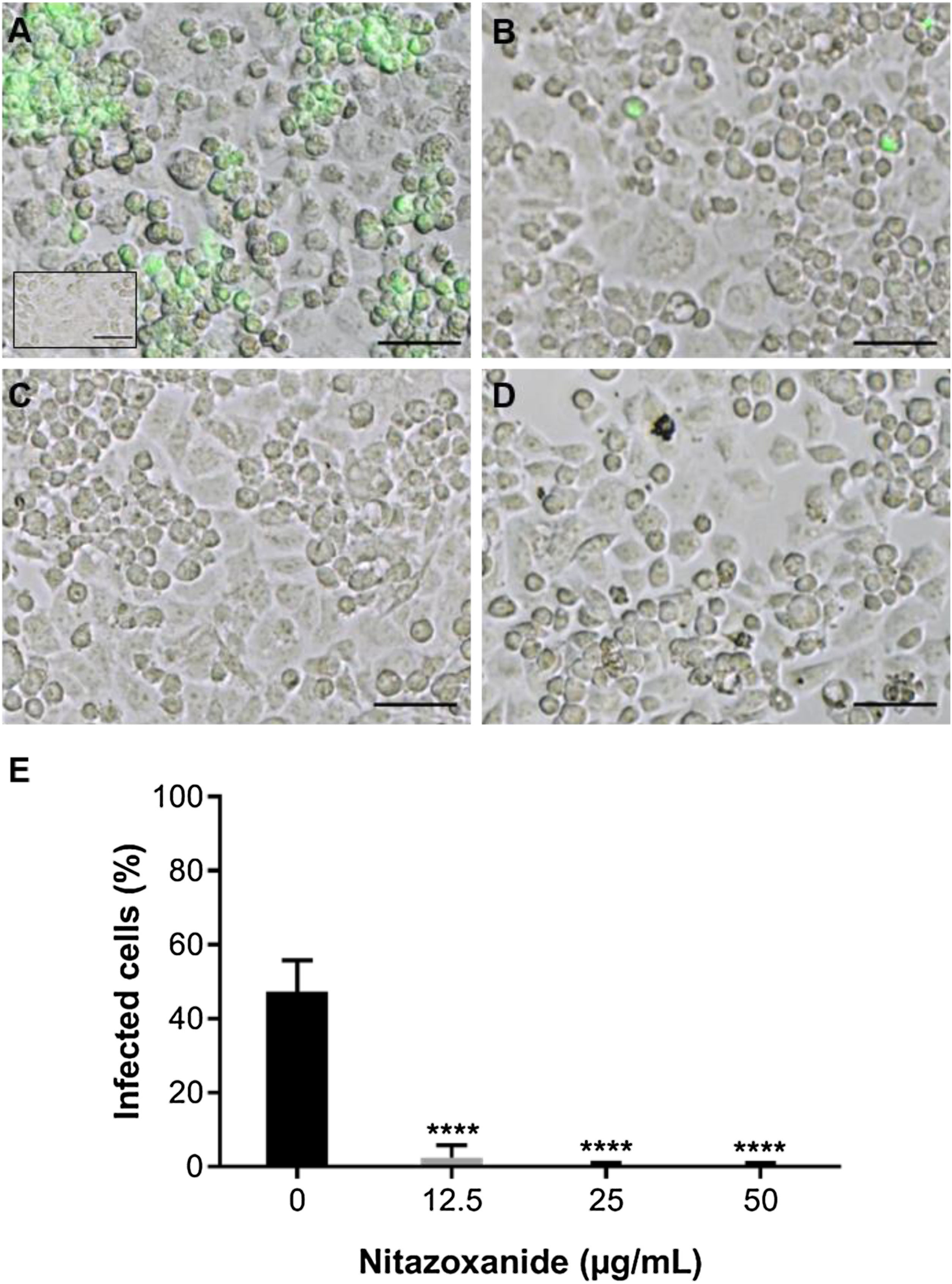
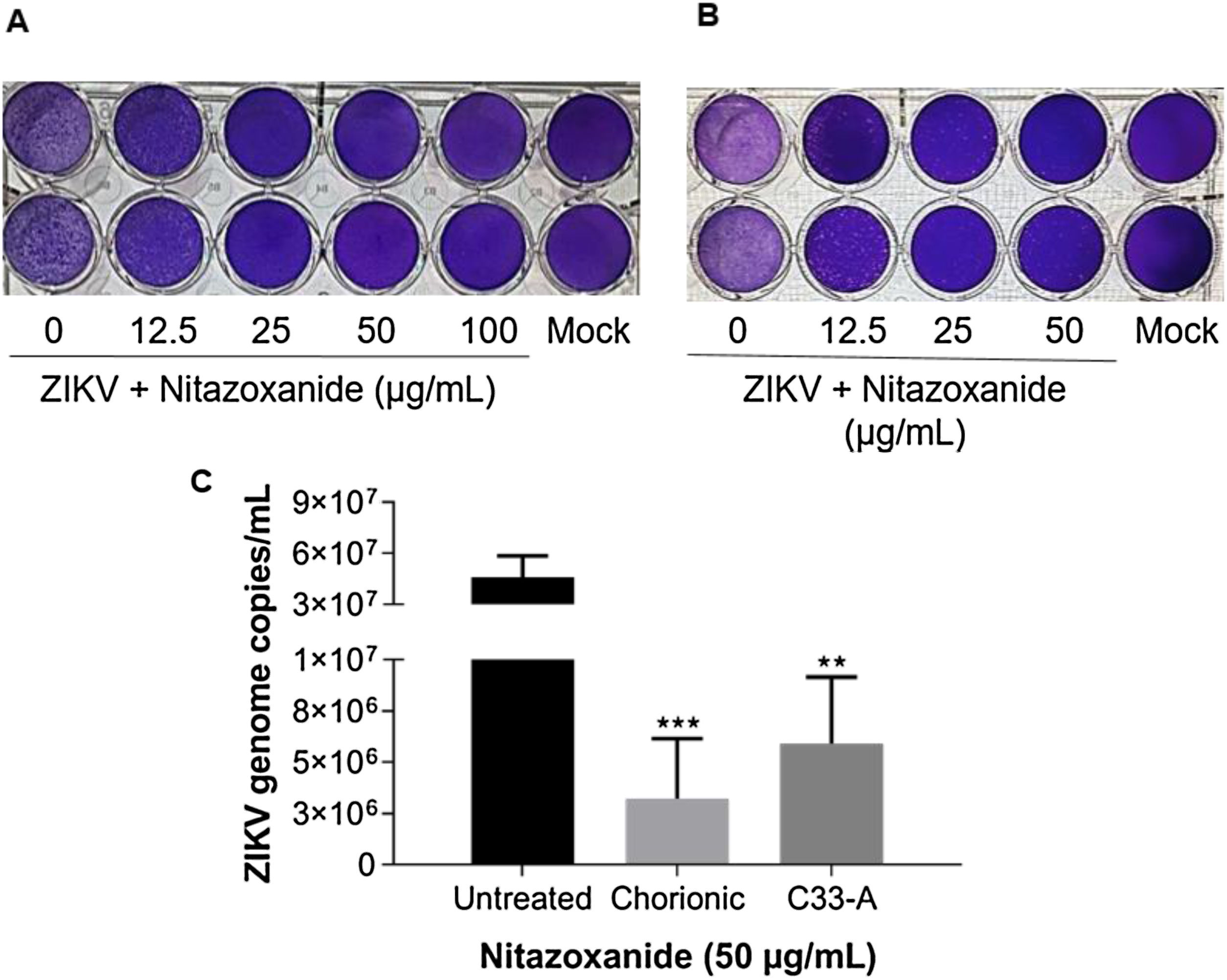
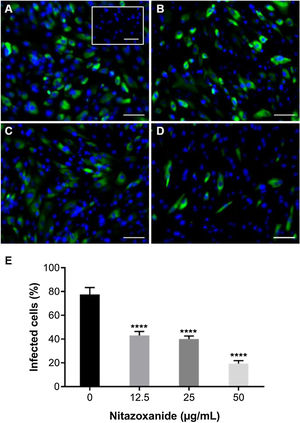
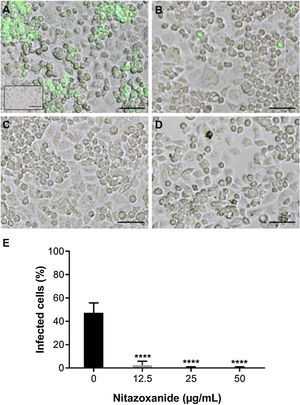
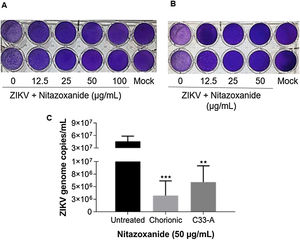
As observed in Vero cell cultures, treatment was effective only after the viral inoculum. This treatment regimen was employed in placental (chorionic) and cervical (C33A) cells cultures and strong antiviral activity was confirmed after 48 h of treatment (Figs. 5–7). The antiviral activity of NTZ in chorionic cells led to a significant dose-dependent reduction in the number of infected cells (Fig. 5, Table 1), as well as in fluorescence intensity at 12.5 μg/mL (Fig. 5 B), 25 μg/mL (Fig. 5C) and 50 μg/mL (Fig. 5D) compared to untreated cells (Fig. 5A). At 50 μg/mL, the reduction of infected cells was of 79.2 ± 3% (Figs. 5D and E, Table 1). Regarding cervical cells, treatment with 12.5 μg/mL exhibited a drastic reduction of 95.3 ± 6.5% in the number of infected cells (Figs. 6B and E, Table 1). At 25 μg/mL (Fig. 6C) and 50 μg/mL (Fig. 6D), only about 0.3 ± 0.5% of infected cells were observed. EC50 values were 17.5 ± 0.1 μg/mL for chorionic cells and 4.1 ± 1.0 μg/mL for C33A cells (Table 1) and SI values were 62.1 and 38.0, respectively (Table 1). In addition, RT-qPCR and plaque forming assays were performed (Fig. 7). Quantification of viral loads in the culture supernatants treated with 50 μg/mL revealed a significant reduction to 3.2 ± 2.8 × 106 copies/mL and 5.9 ± 3.2 × 106 copies/mL in chorionic and cervical cells, respectively, compared with 4.6 ± 1.2 × 107 copies/mL in untreated cultures (Fig. 7C). To confirm these results, the production of infectious viruses by treated cells was analyzed. Of importance, a 25 μg/mL concentration in chorionic cells inhibited 100% of viral progeny (Fig. 7A). However, despite the observed reduction in infectious virus production by the treated cervical cells, complete inhibition was not observed (Fig. 7B).
Antiviral effect of Nitazoxanide on a primary chorionic cell culture determined by immunofluorescence. The cultures were infected or not (inset A) with ZIKV at a MOI of 20 and subsequently treated or not (A) with (B) 12.5 μg/mL, (C) 25 μg/mL or (D) 50 μg/mL of the drug for 48 h. The fluorescence staining shows the viral antigen (green) and the host cell nucleus (blue). (E) The graph represents the means ± standard deviations of the percentage of infected cells. Statistical significance was determined by one-way ANOVA, followed by Dunnett’s multiple-comparisons test. ****p < 0.0001. The data are representative of 3-5 experiments run in duplicate. Bars 100 µm.
Antiviral effect of Nitazoxanide on cervical cells (C33-A). Cultures were infected or not (inset A) with ZIKV at a MOI of 10 and subsequently treated or not (A) with (B) 12.5 μg/mL, (C) 25 μg/mL or (D) 50 μg/mL of NTZ for 48 h. The fluorescence staining shows the viral antigen (green). (E) The graph represents the means ± standard deviations of the percentage of infected cells. Statistical significance was determined by one-way ANOVA, followed by Dunnett’s multiple-comparisons test. ****p < 0.0001. The data are representative of 3-5 experiments run in duplicate. Bars 50 µm.
Evaluation of anti-ZIKV effect of Nitazoxanide on host cells. (A) The primary cultures of chorionic cells were infected or not (Mock) with ZIKV at a MOI of 20 and (B) cervical cells (C33-A) at a MOI of 10 and subsequently treated with 12.5, 25, 50 or 100 μg/mL of NTZ for 48 h. Furthermore, (A and B) the culture supernatants were collected for the detection of infectious viruses by the plaque assay and (C) quantification of viral loads. The graph represents the means ± standard deviations of the copies of the ZIKV genome/mL. Statistical significance was determined by one-way ANOVA, followed by Dunnett’s multiple-comparisons test. **p < 0.0013, ***p = 0.0009. The data are representative of 3–5 experiments run in duplicate.
This study described in vitro models of ZIKV infection in a primary culture of human placental chorionic cells and human cervical epithelial cells to assess antiviral activity of nitazoxanide. Currently, the main concerns related to Zika infection are transplacental transmission and CZS development. Recent studies investigating how ZIKV reaches the fetus have reported virus tropism by the human placenta, including infection of the amniochorionic membrane, trophoblasts, endothelial cells, fibroblasts and Houfbauer cells.11,27,28 In addition, ZIKV sexual transmission can lead to infection of the genital tract and, through cell-cell infection, reach the placenta. Indeed, several in vitro and in vivo studies have shown the permissiveness of cells in the uterus, ovary, vagina and cervix for ZIKV infection.29–31 Although the importance of the infection of these cells has not been fully established, the virus must cross the protective cell layers to reach the fetal compartment.28,32 In this context, due to peculiar conditions of gestational tissue and viral replication sites, the uteroplacental microenvironment is important to consider during the investigation of any potential anti-ZIKV therapeutics, especially for the treatment of infected pregnant women.
In this study, the ZIKV BR, isolate from a Brazilian patient during the 2015 outbreak, was used and experiments were conducted with a low number of viral passages. We observed different infectivity potentials of the Zika virus according to the host cells. Human cells were more resistant to Zika BR infection than non-human primate cells, while insect cells were much more resistant to infection than the other cell types assessed herein (Figs. 2S A–D). Corroborating our data, Thaker et al. demonstrated that ZIKV infection differentially reprograms the host cell glucose metabolism, leading to cell death in human but not in C6/36 mosquito cells.33 Regarding infection rate, studies have reported that ZIKV BR isolates display differential abilities to infect lineages or primary mammalian cells and insect cells.34,35 Moreover, mammalian cells also showed different susceptibility rates when infected with the Asian and African ZIKV lineages, and infection with ZIKV prototypes (MR766 and PE243) induces greater protein expression and viral progeny than ZIKV BR isolates.34 Thus, the increased resistance observed in human cells and the low infectivity potential of ZIKV BR led us to infect them with a high viral load to obtain a high percentage of infected cells and to assess the stringent antiviral effect of NTZ.
The repurposing of NTZ against ZIKV BR and DENV-2 demonstrated potent antiviral activity in mammalian cells and the drastic reduction of progeny virion production. Drug repurposing has emerged as an alternative approach to accelerate drug development, where new indications for existing drugs may be quickly identified.36 NTZ is available in the clinic with extensive experience involving approximately 75 million adults and children.37 In addition to its clinical use for the treatment of rotavirus and norovirus infections, clinical trials are under investigation for the treatment of influenza38 and hepatitis B and C.39,40
This drug also resulted in the experimental inhibition of dengue virus,23 Japanese encephalitis virus41 and coronavirus42 among others virus. The potent property of NTZ in inhibiting the replication of Brazilian ZIKV strain was demonstrated in chorionic, cervical and Vero cells. Corroborating our data, NTZ inhibited the replication of SZ-WIV01 ZIKV strain isolated from an American Samoan patient, in Vero cells22 and in a Puerto Rico PRV-ABR59 ZIKV strain in A549 cells.23 A Vero cell screening assay revealed that NTZ treatment was effective only after the viral inoculum, with the highest antiviral activity observed at 48 h compared to 24 h post-infection. This data suggests that a greater amount of bioactive metabolite of NTZ could be available in cultures at this time of infection. Indeed, when a fresh medium containing NTZ was added every 24 h, the same antiviral effect was not observed (data not shown). In humans, when orally administered, the redox-active nitrothiazolyl-salicylamide prodrug rapidly deacetylates in blood to its active metabolic form, tizoxanide.43 We also observed that the effectiveness of a single-dose treatment was related to the infection rate in Vero cells, evidencing that viral load is a factor to be considered in defining therapeutic management. In fact, studies concerning cytomegalovirus disease report that viral quantification has been used for its prognostication and to guide the need to initiate preemptive therapy and concerning treatment duration.44 A clinical study using viral dynamics models taking influenza A as an example demonstrated that viral load was positively correlated with body temperature and symptom scores, and only when amantadine treatment was given before the peak viral load was a strong impact observed in infection.45
Viral growth parameters (infection rate and viral production rate) indicate that NTZ was highly effective against the ZIKA virus in both human placental chorionic cells and human cervical cells, but with differential therapeutic responses. CC50 values indicate greater resistance to NTZ toxicity in chorionic cells, which may be important as the placental barrier presents physiological specificities involved in pregnancy maintenance and fetal defense. The Cmax of plasma tizoxanide is 10.6 µg/mL with a Tmax of 3 h and an AUCτ of 41.9 µg.h/mL following administration of a 500 mg tablet,46 while, treatment with a single dose of NTZ at different concentrations resulted in an EC50 of 17.5 µg/mL in chorionic cells and 4.1 µg/mL in cervical cells at 48 h pos-infection. It is important to consider that the antiviral effect found in chorionic cells was upon high infection rate (78 ± 11%) and viral load of 4.6 ± 1.2 × 107 RNA copies/mL, while the mean viral load in samples from ZIKV-infected patients were 5 × 104 RNA copies/mL in blood and 2 × 104 RNA copies/mL in urine.26 The selectivity index (SI) values were high for both cells. This parameter is widely accepted to express the in vitro efficacy of a compound in inhibiting virus replication, and the higher the SI value, the more effective and safer a drug would be during in vivo treatment. It should be pointed out that, although the EC50 value was higher for chorionic cells, its SI value of 62 was almost two-fold higher than the value observed for cervical cells. The excellent therapeutic window found, mainly, in placental cells, represents an important finding obtained with NTZ therapy for the first time.
NTZ reduced the infection rate, viral progeny production and RNA accumulation in a dose-dependent manner, although the specific mechanism is still unknown, evidencing a decrease in viral replication. As action mechanisms, NTZ has been shown to deplete intracellular Ca+ storage,47 block of hemagglutinin protein maturation of Influenza A virus at the post-translational level,48 alter the ability of rotavirus and norovirus to replicate efficiently, reducing the size and inhibiting the formation of viroplasm49,50 and, finally, inhibit the interaction of the NS2B-NS3 complex preventing the processing of the flaviviral polyprotein.23 However, further studies should be conducted to identify viral targets and reveal additional NTZ mechanisms of action, which appear to depend both on the virus itself and host cells.37 In fact, NTZ did not inhibit the viral progeny production in C6/36 insect cells (Fig. 2S), and it is possible that the failure of antiviral activity observed in these cells could be related to different NTZ targets involved in viral replication in mammalian and insect cells.
In the present study, infection-related measures demonstrate that NTZ impairs the Brazilian strains of DENV-2 and ZIKV in mammalian cells and that NTZ was highly effective against the ZIKV in both primary chorionic cells and cervical cells. Moreover, the strongest reduction of virion progeny production in chorionic placental cells represents an important result obtained by NTZ therapy for the first time. The high costs for the development of new pharmaceuticals and the excellent in vitro data presented herein should encourage further in vivo research on the repurposing of NTZ against ZIKV infection, keeping the perspective of treating Zika fever, especially in pregnant women. This drug seems to fulfill some key criteria for clinical therapeutic use, namely (i) safety; (ii) low cost; (iii) orally given to children above 1-year-old, adults and during pregnancy, which allows for rapid clinical use in a public emergency.
Ethical approvalThis study was approved by the FIOCRUZ Human Research Ethics Committee under protocol: CAAE 88642218.1.0000. 5269/CEP 2729444).
Conflict of interestsAll authors declare that they have no conflict interests.
The authors would like to thank the Laboratório de Tecnologia de Anticorpos Monoclonais (LATAM/Biomanguinhos/FIOCRUZ) for providing the monoclonal 4G2 antibody, the Laboratório de Flavivirus (LABFLA/IOC/FIOCRUZ) for providing the Zika virus strain and the Laboratório de Tecnologia Virológica (LATEV/Biomanguinhos/FIOCRUZ) for providing the dengue virus strain. This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior- Brasil CAPES, Conselho Nacional de Desenvolvimento Científico e Tecnológico-Brasil CNPq and Fundação Oswaldo Cruz- Brasil FIOCRUZ.