Toxoplasmosis in pregnant women can cause significant morbidity and mortality in the fetus, which may be mitigated by early diagnosis and treatment. Social factors have also been related to the risk of developing the congenital form of toxoplasmosis, since some of these factors interfere directly in the quality of prenatal care. This study aimed to describe the clinical, laboratory, and epidemiological data of pregnant women diagnosed with toxoplasmosis and their newborns followed up at a referral hospital in Rio de Janeiro, Brazil. This was descriptive cohort study of 334 pregnant women with toxoplasmosis followed from May 2014 to December 2017. We conducted interviews to assess knowledge about the disease and its preventive measures, analyzed clinical and laboratory data during antenatal visits, and collected data from the newborns’ medical charts.
ResultsThis was a predominantly low-income women cohort study, with little schooling, mainly referred from public health services late in pregnancy (178; 53.3%), in the second and third trimesters (286; 85.6%). Diagnosis of acute toxoplasmosis had not been confirmed in 171 cases (51.2%). Out of 183 (54.9%) women who had initiated treatment at the original health services, 45 (24.6%) received an incorrect prescription. Seventy-two amniocenteses were performed, with positive real-time polymerase chain reaction (qPCR) in the amniotic fluid in two cases (2.8%). Congenital toxoplasmosis at birth was identified in eight newborns (5.4%).
ConclusionLate referral to specialized medical services, inadequate toxoplasmosis management at the original prenatal care services, and social vulnerabilities are contributing factors to the persistent occurrence of congenital toxoplasmosis cases.
Toxoplasmosis is a zoonosis of worldwide distribution caused by the protozoan Toxoplasma gondii.1 It is problematic when acquired during pregnancy, due to the risk of vertical transmission.2 The infection is usually asymptomatic, including during pregnancy,3 so serological screening in routine prenatal care is essential for early diagnosis and identification of susceptible pregnant women.4
Cases of congenital toxoplasmosis can evolve with miscarriage, neurological and visual abnormalities, or be asymptomatic at birth developing late clinical manifestations.5 In Brazil, an estimated 6,000–9,000 cases of congenital toxoplasmosis occur per year.1 More virulent toxoplasma strains identified in South America have been associated with more severe cases.1
Congenital infection is confirmed by serology with identification of IgG and IgM antibodies against T. gondii and the avidity test,4 based on detection of toxoplasma DNA in the amniotic fluid by polymerase chain reaction (PCR).6 The latter test has high sensitivity (87%) and specificity (99%), contributes to diagnosis of fetal infection, and reduces unnecessary treatment with sulfadiazine, pyrimethamine, and folinic acid.7 Early diagnosis and prompt and adequate treatment reduce future damage to the fetus.8
Social factors have also been related to the risk of developing congenital toxoplasmosis, since some factors interfere directly in the quality of prenatal care, with late collection of serological samples, delay in starting treatment, and delay in referral to follow-up at treatment centers.9
Despite the importance of this disease, there is still no international consensus on its surveillance in pregnant women.10 The Brazilian Ministry of Health recommends serological screening during prenatal care, but thus far the municipal health services are responsible for defining the best strategy to be adopted.11
This study aims to describe the clinical, laboratory, and epidemiological characteristics of a population of pregnant women with toxoplasmosis followed up at a referral hospital in Rio de Janeiro.
Material and methodsThis was a descriptive cohort study of pregnant women followed up at the outpatient clinic for infectious and parasitic diseases at the National Institute of Health of Women and Children Fernandes Figueira (IFF/Fiocruz) from May 2014 to December 2017, referred with a diagnosis of toxoplasmosis. Pregnant women with HIV, hepatitis B and C co-infection, and on any type of immunosuppressive therapy were excluded.
Sociodemographic data, previous knowledge of the disease and its prevention, and clinical, laboratory, and sociodemographic data were collected during medical appointments according to a protocol and conducted by the same researchers.
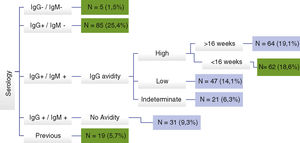
Serological diagnosis was performed with an immune fluorometric assay, or enzyme-linked fluorescent assay (ELFA). All participants underwent this test at the referral hospital, regardless of whether they had previous serological results in other institutions. Acute toxoplasmosis was defined according to the following criteria: (i) anti-T. gondii IgM and IgG positivity with low IgG avidity in cases with gestational age (GA) less than or equal to 16 weeks and (ii) IgM and IgG positivity regardless of IgG avidity in cases of GA greater than 16 weeks.
Amniocentesis for molecular diagnosis of fetal infection was offered to pregnant women with confirmation of acute infection by toxoplasma, with GA between 16 and 31 weeks.
Amniotic fluid samples were sent to the High Complexity Laboratory of the IFF/Fiocruz, where molecular diagnosis of toxoplasmosis was performed with real-time polymerase chain reaction (qPCR) using the TaqMan® Real-Time PCR system. The 529 base pair fragment was chosen as a target gene. Primers 318R and 270 F were used, and the probe, the sequence of which was described by Robert-Gangneux et al.12
For qPCR reactions, 5 µl of DNA extracted from the pregnant woman’s amniotic fluid was used, to which 1 µl of primer-probe mix was added, containing primers and probe at concentrations of 1.5 μM and 1.25 μM, respectively; 1.0 µl of internal control (20x RNAseP Primer Probe, Applied Biosystems) and 7.5 µl of TaqMan® Real-Time PCR Master Mix, for a final volume of 15 µl.
A positive control (parasite DNA) and negative control with no DNA sample were included. All reactions with patient DNA and positive and negative controls were performed on the Applied Biosystems 7500 Real-Time PCR equipment. The cycling included a first stage (50 °C for 2 min, 10 min at 95 °C) and a second stage (40 repetitions of the following cycle: 15 s at 95 °C and 1 min at 60 °C). At the end of the run, data analysis was performed using Applied Biosystems 7500 Real-Time PCR Software, generating amplification graphs and tables with each sample’s Ct values.
Data on newborns were obtained by telephone contact within 60 days after the expected delivery date and through analysis of medical records of those born in the same institution. The following data were collected from the newborns: transfontanelle ultrasonography, fundoscopy, gestational age and birthweight, and serology for toxoplasmosis IgM and IgG in peripheral blood on the fifth day of life.
Diagnosis of congenital toxoplasmosis was established according to the criteria published in Pediatrics (2017)13: (i) positive PCR in the amniotic fluid with or without intrauterine ultrasound changes suggestive of congenital toxoplasmosis and serological evidence of acute infection by T. gondii in the gestation; (ii) asymptomatic cases at birth, with reactive IgM and IgG serology at more than five days of age, but clinical, radiological and laboratory tests without abnormalities; (iii) newborns symptomatic at birth, with reactive IgM and IgG serology and with any clinical, radiological, or laboratory alteration suggestive of congenital toxoplasmosis.
The Epi Info program version 7.2 was used. In the exploratory data analysis, categorical variables were described as absolute and relative frequencies, and numerical variables were presented as means.
The local Institutional Review Board approved the study (case review 3.587.173). All study participants were informed verbally and in writing and signed the free and informed consent form.
ResultsThe study sample included 334 participants with a median age of 27 years (interquartile 25% of 21 and 75% of 32 and minimum of 12 and maximum of 46 years). One hundred and seventy-eight women (53.3%) were referred from public health services, and 166 (49.7%) reported the current as their first pregnancy.
Of a total of 334 pregnant women, 246 (73.6%) answered the sociodemographic questionnaire, which also included questions related to knowledge of the disease.
The majority, 186 (75.6%), had a family income of up to three minimum wages, 51 (20.7%) had 16 or more years of schooling, and 87 (35.4%) had private health insurance. More than half of the women lived outside the city of Rio de Janeiro (138/56.1%), and in 149 (60.6%) cases, the pregnancy was unplanned.
Regarding knowledge of toxoplasmosis, 171 women (69.5%) were unaware of the disease before their own diagnosis. Eighty-two of the women (33.3%) obtained information from their attending physicians in their home services. Sixteen (6.5%) had no prior information, and 103 (41.9%) sought other sources of information, mainly digital media. Before consultation at the referral hospital, 205 of the women (83.3%) reported that they had never been informed of the existence of amniocentesis as a diagnostic procedure for fetal infection.
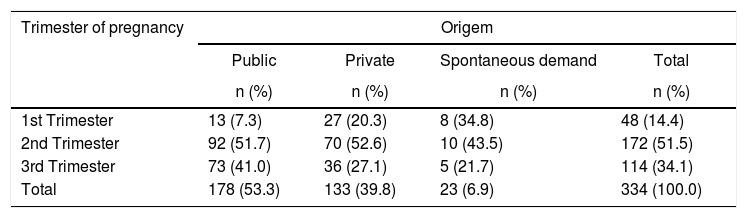
Among the 334 patients seen at the referral hospital, 286 (85.6%) had the first visit in the second and third trimesters of pregnancy. Among those seen in the first trimester, 27 (56.2%) were referred by private services, 13 (27.1%) by public services, and eight (16.7%) on their own initiative. Despite the delay to reach the referral hospital, 162 (48.5%) participants collected toxoplasmosis serology in the first trimester and no significant differences was observed in the prenatal care provided in the public and private services, as in both cases there was a referral delay to the reference center (Table 1).
Pregnancy trimester at first antenatal care visit in IFF/Fiocruz and health system referral pattern.
| Trimester of pregnancy | Origem | |||
|---|---|---|---|---|
| Public | Private | Spontaneous demand | Total | |
| n (%) | n (%) | n (%) | n (%) | |
| 1st Trimester | 13 (7.3) | 27 (20.3) | 8 (34.8) | 48 (14.4) |
| 2nd Trimester | 92 (51.7) | 70 (52.6) | 10 (43.5) | 172 (51.5) |
| 3rd Trimester | 73 (41.0) | 36 (27.1) | 5 (21.7) | 114 (34.1) |
| Total | 178 (53.3) | 133 (39.8) | 23 (6.9) | 334 (100.0) |
Thirty-six pregnant women (22.1%) with confirmed acute toxoplasmosis had symptoms suggestive of primary infection, mainly fever, headache, lymph node enlargement, asthenia, myalgia, and sore throat.
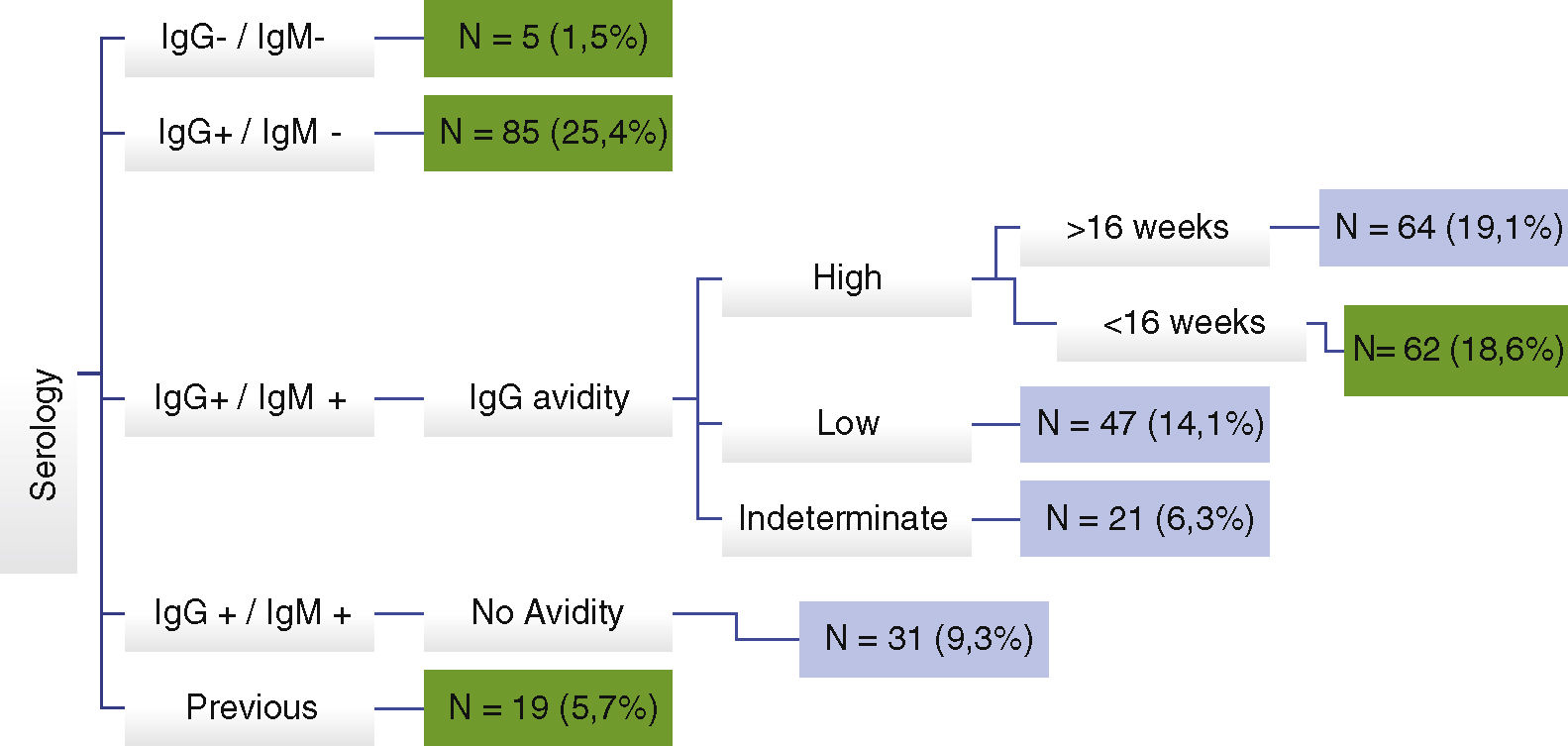
Diagnosis of toxoplasmosis during pregnancy was not confirmed in 171 (51.2%) cases for the following reasons: non-reactive IgM with reactive IgG; reactive IgM and IgG with high IgG avidity before 16 weeks of gestational age; and false-positive IgM, when serology was repeated at IFF/Fiocruz. IgG avidity was not performed at the source in 218 participants (65.3%) (Fig. 1).
In 183 of the women (54.9%), treatment was started at the original health service. However, it was prescribed incorrectly in 45 cases (24.6%), mostly with incorrect dosage. Among the 163 cases (48.8%) of acute toxoplasmosis confirmed at IFF/Fiocruz, 49 (14.7%) received sulfadiazine, pyrimethamine, and folinic acid, 113 (33.8%) received spiramycin, and one (0.3%) received clindamycin, pyrimethamine, and folinic acid. Adverse events occurred in four women who received sulfadiazine, requiring medication change in two of these cases. Four women presented adverse events with the use of spiramycin, requiring medication change in one. The use of clindamycin was justified by a previous report of sulfa allergy.
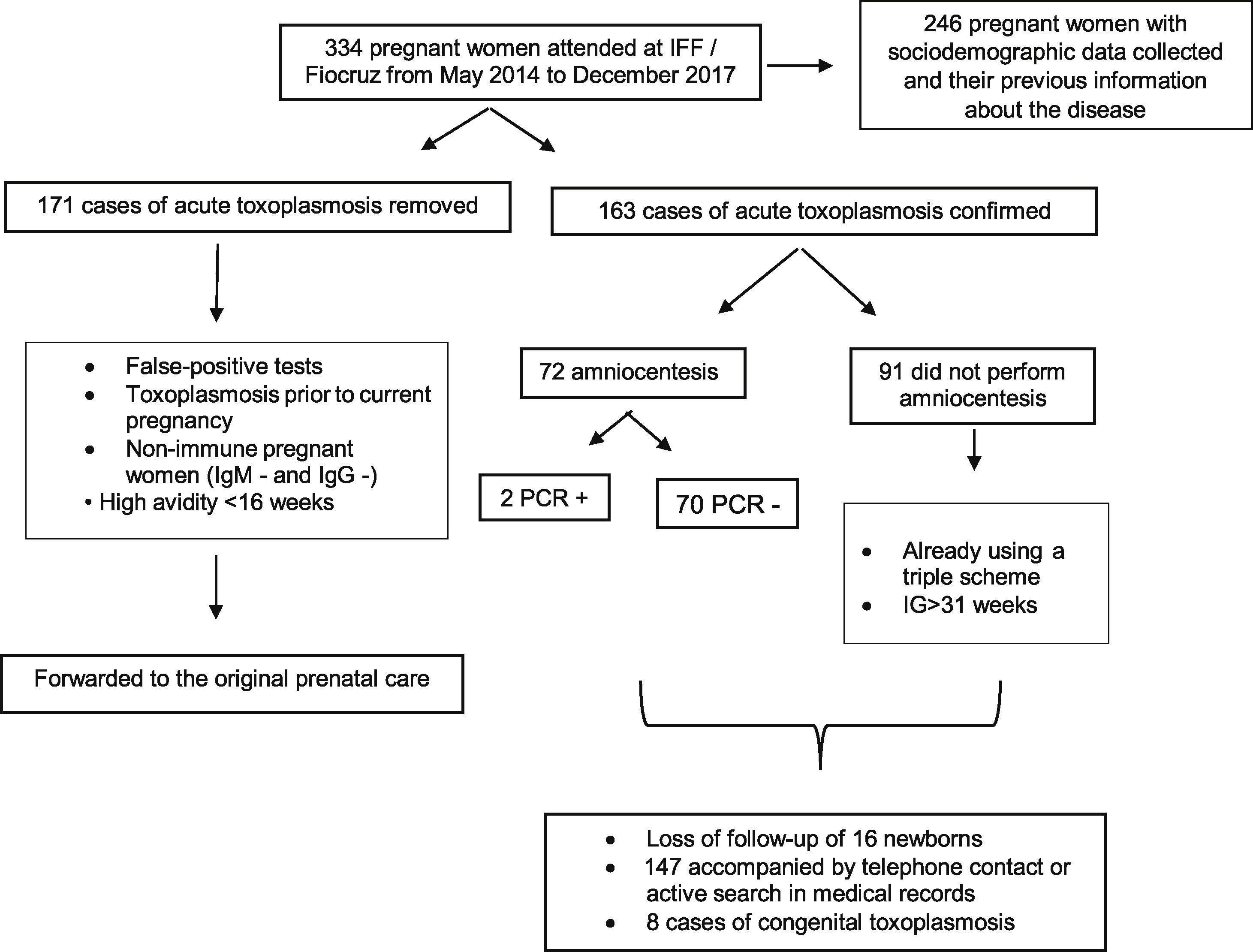
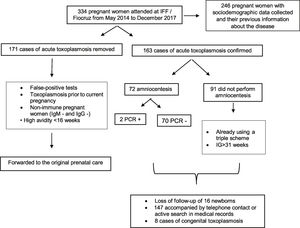
Amniocentesis was performed in 72 women (21.6%) with mean gestational age of 23 weeks (minimum 16 and maximum 31 weeks). In two cases (2.8%), qPCR was positive in the amniotic fluid. One patient was asymptomatic, and in both morphological ultrasound tests performed before the procedure there were no changes suggestive of congenital infection. Only one case presented fetal morphological changes, but qPCR was negative. The patient had received the regimen with sulfadiazine, pyrimethamine, and folinic acid for 12 days before amniocentesis, but the treatment had only started at gestational age of 30 weeks. Diagnosis of congenital toxoplasmosis was confirmed in the newborn based on ocular and neurological lesions. The relatively low number of amniocentesis tests (72) was not because of patients’ refusal to undergo the procedure. Only one patient declined amniocentesis when offered. The main reason for not performing amniocentesis in a significant number of cases was advanced gestational age at which patients were admitted to the referral hospital (mainly in the third trimester) (Fig. 2).
There were no cases of abortion secondary to toxoplasmosis infection, nor were there any complications secondary to amniocentesis.
Information on newborns by telephone contact or active search for data in the medical records of those born at the maternity hospital of IFF/Fiocruz were obtained in 147 (90.2%) of the 163 pregnancies with confirmed diagnosis of toxoplasmosis.
There were 16 cases lost to follow-up, mainly due to difficulty in making telephone contact with women whose children had not been born at the IFF/Fiocruz maternity hospital.
Mean gestational age at birth was 39 weeks (minimum of 33 and maximum of 42 weeks), with 11 of infants (7.5%) born at less than 35 weeks. Mean birthweight was 3280 kg (minimum 1485 and maximum 4055 kg).
Transfontanelle ultrasound was altered in three newborns (2.0%), and fundoscopy in three (2.0%). Six newborns (4.1%) presented positive IgM for toxoplasmosis in the peripheral blood after the fifth day of life. In 122 cases (83.0%), IgM was not detected, and in 19 (12.9%) it was not performed. IgG was positive in 93 newborns (63.3%), negative in 31 (21.1%), and not performed in 23 (15.6%).
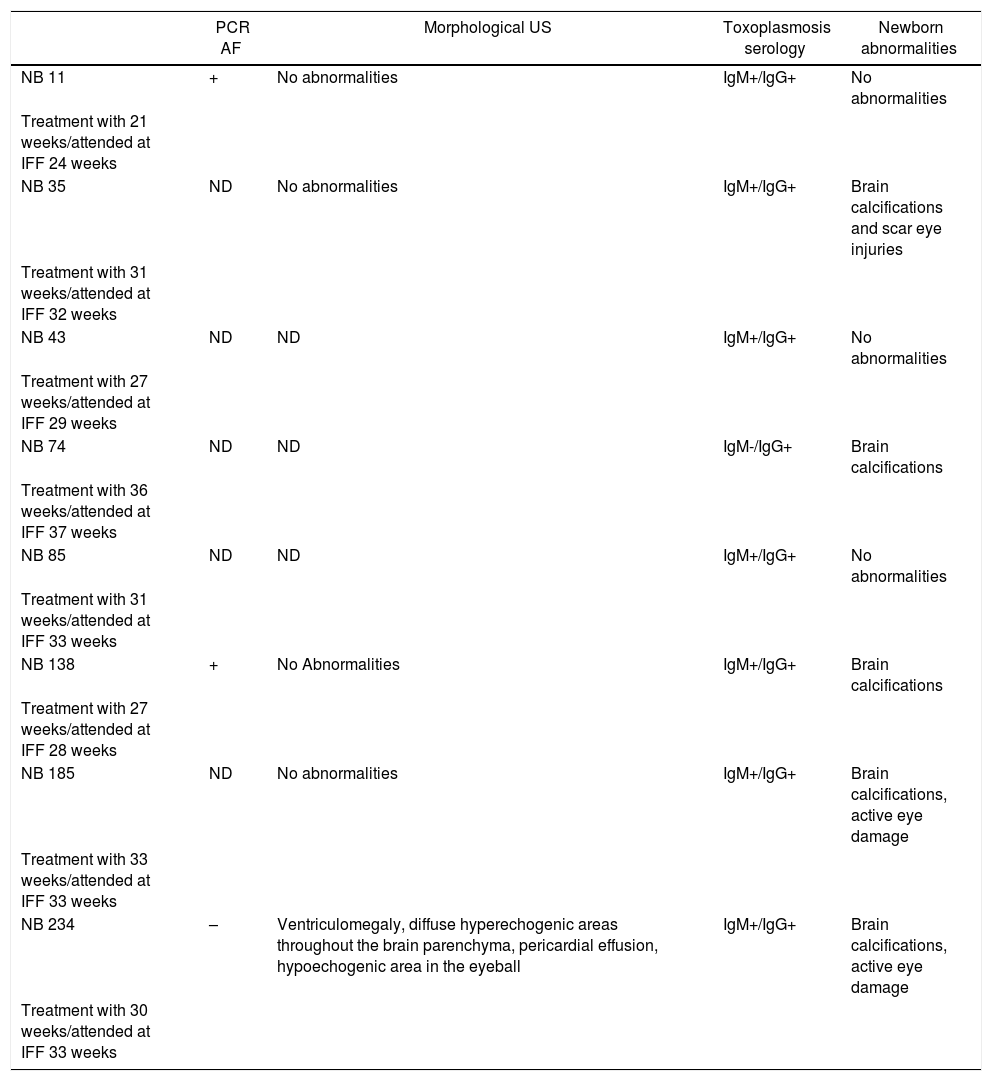
Among the 147 newborns whose information at birth we were obtained, eight (5.4%) were diagnosed with congenital toxoplasmosis. Of these, treatment was initiated in five mothers in the third trimester and in three in the second trimester (Table 2). Four cases were mothers who seroconverted in the third trimester.
Description of newborns diagnosed with congenital toxoplasmosis.
| PCR AF | Morphological US | Toxoplasmosis serology | Newborn abnormalities | |
|---|---|---|---|---|
| NB 11 | + | No abnormalities | IgM+/IgG+ | No abnormalities |
| Treatment with 21 weeks/attended at IFF 24 weeks | ||||
| NB 35 | ND | No abnormalities | IgM+/IgG+ | Brain calcifications and scar eye injuries |
| Treatment with 31 weeks/attended at IFF 32 weeks | ||||
| NB 43 | ND | ND | IgM+/IgG+ | No abnormalities |
| Treatment with 27 weeks/attended at IFF 29 weeks | ||||
| NB 74 | ND | ND | IgM-/IgG+ | Brain calcifications |
| Treatment with 36 weeks/attended at IFF 37 weeks | ||||
| NB 85 | ND | ND | IgM+/IgG+ | No abnormalities |
| Treatment with 31 weeks/attended at IFF 33 weeks | ||||
| NB 138 | + | No Abnormalities | IgM+/IgG+ | Brain calcifications |
| Treatment with 27 weeks/attended at IFF 28 weeks | ||||
| NB 185 | ND | No abnormalities | IgM+/IgG+ | Brain calcifications, active eye damage |
| Treatment with 33 weeks/attended at IFF 33 weeks | ||||
| NB 234 | – | Ventriculomegaly, diffuse hyperechogenic areas throughout the brain parenchyma, pericardial effusion, hypoechogenic area in the eyeball | IgM+/IgG+ | Brain calcifications, active eye damage |
| Treatment with 30 weeks/attended at IFF 33 weeks |
AF = amniotic fluid /US = ultrasound/ND = not done.
Referral delay to the tertiary hospital, lack of information on the disease provided to patients, and no initiation of treatment at the original health services indicate difficulties in the prenatal care provided to these pregnant women. A similar scenario has been described previously,9 suggesting persistent problems in prenatal care and indicating missed opportunities for congenital toxoplasmosis prevention.
Although the study sample included mostly low-income women with little schooling and no private health insurance, the quality of care provided in public and private services was not significantly different. Both delayed referral to the tertiary hospital and initiation of treatment, problems that have been reported by other authors.9
Even with the majority reporting previous pregnancies, 69.5% of these women were unaware of toxoplasmosis before their own diagnosis. A significant proportion sought information on digital media without any guidance from their primary prenatal care professionals. Besides, most participants had never heard of the amniocentesis test. Similar results were reported by another Brazilian study with 2136 pregnant women, 93% of whom were unaware of toxoplasmosis.14 These findings may reflect both the low quality of information provided to pregnant women and inadequate trainning of health professionals to deal with toxoplasmosis in pregnancy.
Symptoms suggestive of acute toxoplasmosis were observed in 22.1% of cases, consistent with reports in the literature,3 which varies from 10% to 30%.10,15 This emphasizes the importance of prenatal serological screening for toxoplasmosis,3 but as there is still no national serological screening policy in Brazil for toxoplasmosis in prenatal care,11 with lost opportunities for both diagnosis and prevention.
The immunological diagnosis of toxoplasmosis in pregnancy has some limitations.4 False-positive results and the presence of residual IgM result in difficulties in interpreting the tests.16 Remarkably, in 51.2% of cases, the diagnosis of acute toxoplasmosis was ruled out. In these cases, there were inconsistent results between the tests performed at the original health service and those performed at the referral service or inadequate interpretation of the results, corroborating a previous study.9 However, in cases of doubt regarding the interpretation of results and management, the need for referral and evaluation in tertiary hospitals has already been suggested by other authors.17
The IgG avidity test is mainly useful in an appropriate time frame (less than 16 weeks gestational age).4 This test was not performed in 65.3% of patients in the current sample, thus missing an essential diagnostic tool that could reduce the emotional impact because of the possibility of diagnosing a congenital disease and the unnecessary use of more complex and expensive diagnostics, treatments and procedures.
In almost half of the cases (45.0%), treatment was not initiated at the original health service, and inadequate dosage was seen in another 24.6% of the cases. This treatment-related difficulty had been previously reported in Rio de Janeiro, where treatment was started in only 16% of cases and incorrect doses prescribed in 5%.9 Lack of knowledge and experience with toxoplasmosis by professionals providing prenatal care have been observed in the United States18 and in another Brazilian study.17
Testing of amniotic fluid by qPCR is an excellent tool for confirming the diagnosis of fetal infection after the 18th week of gestation.7 Its high sensitivity and specificity contribute to early (intrauterine) diagnosis of fetal infection and reduces unnecessary use of sulfadiazine, pyrimethamine, and folinic acid, which has been associated with life-threatening adverse events.19 Considering the positivity of PCR in only two cases, this treatment was avoided in 70 negative cases.
Surprisingly, IgG against T. gondii was not detected in 31 (21.1%) newborns, probably associated with low levels of maternal antibodies, IgG subclass, gestational age, or impaired placental integrity.20
No complications of amniocentesis were observed, which reinforces safety of the procedure when performed by a qualified professional. In Brazil, until now, amniocentesis is not routinely recommended via a protocol from the Ministry of Health, and its availability is restricted to referral hospitals.21
In the case with negative PCR where diagnosis of congenital toxoplasmosis was confirmed, the woman was already taking sulfadiazine, pyrimethamine, and folinic acid 12 days before collection of amniotic fluid, and the medication may have interfered with the PCR result.22
Diagnosis of toxoplasmosis during pregnancy was not associated with prematurity or low birthweight. Eight newborns were diagnosed with congenital toxoplasmosis according to international criteria.23 Three of them had amniocentesis performed, but in the other five, amniocentesis was not performed, due to advanced gestational age, but treatment was prescribed immediately at the first appointment.
The study's limitations feature the possibility of recall bias when mothers were contacted by phone in cases where delivery occurred outside the referral hospital, thus without the possibility of accessing medical records.
Late referral to the tertiary hospital, inadequate case management by the original health services regarding diagnosis and treatment of pregnant women with toxoplasmosis, and socioeconomic difficulties experienced by these patients contribute for continued occurrence of congenital toxoplasmosis. Lack of a clinical protocol to guide health professionals leaves room for conflicting approaches.
Insufficient knowledge about the true burden of toxoplasmosis in pregnancy may help explain why congenital toxoplasmosis continues to be a largely neglected disease.3 This study of a cohort of pregnant women treated at a referral hospital may be useful for identifying the main problems in the management of these cases and contribute to policies for the prevention of congenital toxoplasmosis.
Authors' contributionsBBFV contributed to the clinical diagnosis of patients and collected samples, implemented the research, analyzed the results, and wrote the manuscript. VCL, JFL, and JPPJ clinically diagnosed patients and collected samples. DNR processed the experimental data. LHFG processed the experimental data, analyzed the results, and wrote the manuscript. SCGJ conducted the statistical analyses. ESN and LCG designed the study and were responsible for the overall direction of the project. All authors discussed the results and contributed to the final manuscript.
FundingThis research was funded by the “Research Incentives Program I (PIP I)” and approved by the Research Department of the Fernandes Figueira Institute (IFF/FIOCRUZ).
Ethics approval and consent to participateThe study was approved by the local Institutional Review Board (CAAE: 148327.13.7.0000.5269/Case Review Number: 2.482.189). Written informed consent terms was obtained from all participants in this study and from the consent of the LAR or parents or guardians of the minors. All experimental protocols in this manuscript were carried out in accordance with the ethical principles that govern research with human subjects, in accordance with the guidelines of the Declaration of Helsinki
Conflict of interestThe authors declare no conflicts of interest.
Availability of data and materialsThe datasets generated and/or analyzed during the current study are not publicly available due to the confidentiality and ethical aspects related to patient data, but are available from the corresponding author on reasonable request.
The authors with to thank the patients and their families for participating in the study. All the experiments were developed in the technological platform “RPT09I – PCR in Real Time – IFF, belonging to the FIOCRUZ Network of Technical Platforms (PDTIS/FIOCRUZ).