The aim of this study was to investigate the immunomodulatory properties of cell wall extract from Enterococcus faecalis CECT7121, measuring the induction of cytokines TNF-α, IL-6, IL-10 and IL-12 from human peripheral blood mononuclear cells (PBMCs). Cell wall extract was prepared from their growth in brain heart infusion broth (18h, 35°C). Subsequently, toxicity of the obtained cell wall extract was tested in Balb-C mice. PBMCs were isolated from buffy coats at the Blood Transfusion Service of Hospital Ramón Santamarina (Tandil, Argentina). PBMCs were purified using standard Ficoll-Paque gradient centrifugation. Aliquots of purified leukocytes were incubated at 37°C for 24h with heat-killed E. faecalis CECT7121 and cell wall extract. Concentrations of IL-6, TNFα, IL-10 and IL-12 (p70) were measured by solid phase sandwich ELISA. Changes in appearance and behavior of mice were evidenced only in the group with the maximal concentration of wall cell extract used (10,000μg). Cell wall extract and heat-killed E. faecalis CECT7121 induced the production of significantly higher amounts of Il-12, IL-6, TNF-α and IL-10 cytokines compared to the nonstimulated PBMCs. These findings provide helpful information on immunomodulation activity by cell wall extract in sight of the application of this compound in controlling certain infectious diseases.
Emergence of multi-drug resistant strains and infectious disease outbreaks caused by resilient strains are the most important global issues related to treatment of invasive infections.1An immune response imbalance can occur in systemic infections; therefore it is important to achieve a balance between pro- and anti-inflammatory cytokines.2
Although inflammation performs a protective function in controlling infection, it can also cause tissue damage and disease. The probiotic approach represents an alternative strategy for prevention and treatment of inflammatory diseases. Probiotic microorganisms can act as modulators of the systemic and mucosal immune responses. Previous studies have shown that different bacterial strains can exert their probiotic properties by influencing the host's immune system, thereby modulating immune responses. It has been proven that probiotic strains of lactic acid bacteria enhance the phagocytic and NK activities and induce maturation of dendritic cells.3
Though therapeutic use of probiotics has been observed for more than a century, safety issues have not been definitively clarified yet. There are case reports of complications with specific bacteria therapy; this suggests the possibility of considering a revision of probiotic safety aspects.4–6 Otherwise, in individuals with a pre-existing structural heart disease or severe immunodeficiency, lactobacilli can translocate and cause severe infection.7
In contrast, non-viable microorganisms or microbial cell extracts could eliminate shelf-life problems and reduce the risks of microbial translocation and infection. Non-viable material of microbial origin can interact directly with the host through the capacity of human cells to recognize specific bacterial components or products, giving rise to responses that involve the immune system. Specialized conserved pattern recognition receptors on host cell membranes, such as Toll-like receptors (TLRs) and the nucleotide-binding domain proteins (NOD), are the primary sensors of the innate immune system and recognize microbe-associated molecular patterns, including peptidoglycan. Previous studies have shown that cell-wall components of lactic acid bacteria stimulate the immune system through interaction with the leukocyte pattern recognition receptors, TLRs. Lipopolysaccharide (LPS) of Gram-negative bacteria induces production of pro-inflammatory cytokines tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6), as well as IL-10, which is known to inhibit the synthesis of the former two cytokines. These pro-inflammatory cytokines contribute to defense mechanisms of the host in response to bacterial colonization or invasion, and when secreted in excess, they may induce pathological disorders of immune origin. IL-12 and IL-10 are important immunomodulatory cytokines produced by cells of the innate immune system. Both compounds are secreted by monocytes/macrophages in response to bacterial products which have opposite effects on the immune system. Functional IL-12 is a heterodimer of 70kD (p70) formed by the covalent union of two chains denominatedp40 (40kD) and p35 (35kD). IL-12 activates cytotoxicity and gamma interferon secretion by T cells and NK cells, whereas IL-10 inhibits these functions.8
In recent years, a non-pathogenic strain, Enterococcus faecalis CECT7121, has been proposed as immunomodulator. When peritoneal macrophages were stimulated with heat-killed E. faecalis CECT7121, synthesis of IL-6, TNFα and IL-12 were induced in a concentration-dependent fashion, whereas synthesis of IL-10 was induced only by the highest concentration 5.0×107CFU/mL of E. faecalis CECT7121. When these cells were stimulated with Salmonella serotype Enteritidis, significantly higher levels of TNFα, IL-6, IL-10 and IL-12 were detected than in the control cells. E. faecalis CECT7121 modulates the innate immune system response by inducing the synthesis of IL-12 and IL-10; two crucial cytokines for the maintenance of the host's immune homeostasis.9–11
In other in vivo experiments, using BALB-C mice, the adjuvant properties of E. faecalis CECT7121 have been demonstrated, as well as its influence on the Th1/Th2 polarization during the specific immune response generated by the immunization with the vaccine DTPw, diphtheria, tetanus and whole-cell Bordetella pertussis.12
The aim of this study was to investigate the immunomodulatory properties of cell wall extract from E. faecalis CECT7121, measuring the induction of cytokines TNF-α, IL-6, IL-10 and IL-12 from human peripheral blood mononuclear cells (PBMCs).
In order to obtain bacterial suspensions, E. faecalis CECT7121 was grown in brain heart infusion broth (Laboratorio Britania, Argentina) at 35°C for 18h. After incubation, cultures were harvested by centrifugation (10,000×g, 15min) and extensively washed with sterile saline solution. Standard growth curves were prepared by plotting the OD620vs. agar plate counts of serially diluted cultures. Bacterial suspensions were prepared in RPMI (Gibco, US) plus 10% fetal calf serum (Gibco, US) for PBMCs experiments. Aliquots of these suspensions were frozen and stored at −70°C until used. For PMBCs experiments, a 5.0×107CFU/mL-heat-killed E. faecalis suspension was selected in agreement with Castro et al.9 Extraction of cell wall from E. faecalis CECT7121 was carried out as follows: a fresh bacterial culture in brain heart infusion broth (35°C, 18h) was submitted to centrifugation at 10,000×g for 15min and then washed with sterile saline solution. Bacteria were suspended in 25mM Tris–HCl, pH 7.4 with 1mM EDTA-Na2 pre-cooled and were submitted to five sonication cycles for 15min each, with 2min intervals on ice in a sonicator 1510 (Braun Biotech Inc., US) at 100W output. Cells were separated by centrifugation at 12,000×g for 10min, and suspended in 25mM Tris–HCl (pH 7.4), 8mM MgCl2, 1μg/mL of RNase A (Sigma, USA) and 1μg/mL of DNase I (Sigma). The sample was incubated at 37°C for 3h, washed twice, and suspended in saline solution. Afterwards, it was heated to 100°C with 1.0(wt.%/vol.%) sodium dodecyl sulfate (SDS) for 4h, centrifuged three times at 12,000×g for 10min, dialyzed against 1L of 1% bovine serum albumin in distilled water, and resuspended in sterile saline solution. Obtained material was lyophilized; dry weight was determined and thenceforth called PEF7121.
Potential toxicity of PEF7121 was evaluated using BALB-C mice (n=8/group) injected intraperitoneally (IP) with 0.5mL of the following amounts of PEF7121; group A: 1000μg, group B: 2000μg, group C: 5000μg and group D: 10,000μg. Control animals were inoculated with 0.5mL sterile saline solution. Clinical appearance of mice was monitored after the challenge with PEF7121 and subjective aspects of behavior were recorded according to the General Health Score scale which takes into account the criteria of Gill et al.13 Briefly appearance and behavior aspects are as follows: S5: bright eyed and alert, a smooth coat with a sheen, responds to stimulus, shows interest in environment; S4: fur slightly ruffled, a loss of sheen of the coat, mouse remains alert and active; S3: fur noticeably ruffled, parts of coat form clumps, not as alert or active, less interested in environment outside of cage, signs of hyperventilating when handled; S2: hunched over and lethargic, little interest shown in environment, fur clumped; and S1: nonreactive to stimulus, fur has a “bottle brush” appearance (i.e., standing on end), hunched over preferring to sleep than react to environment, cold at touch, paws cold. The animals were observed along for 15 days.
PBMCs were isolated from buffy coats obtained from the Blood Transfusion Service of the Hospital Ramón Santamarina (Tandil city, Argentina). To minimize inter-individual variation, all experiments were performed with cells obtained from four blood donors. The study was performed in accordance with the Declaration of Helsinki and the local Ethics Committee.
PBMCs were purified using standard Ficoll-Paque gradient centrifugation according to the instructions of the manufacturer (Amersham Biosciences, Piscataway, USA). After washing, the cells were resuspended in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum, 2mM l-glutamine (Sigma, USA), 100U/mL of penicillin, and 100mg/mL of streptomycin (Gibco, USA) at a final concentration of 106cells/mL. Aliquots (2mL) of purified leukocytes (106cells/mL) were incubated in 24-well plates at 37°C for 24h with stimulators, in the presence of 5% CO2. Heat-killed E. faecalis CECT7121 (5.0×107CFU/mL) and PEF7121 (at the minimal nontoxic concentration resulting from the in vivo experiment) were used to stimulate PBMCs. Purified lipopolysaccharide (LPS) from Escherichia coli O111:B4 (Sigma, USA) was used at a concentration of 1mg/mL as a positive control. Nonstimulated PBMCs were also evaluated as controls of basal cytokine production. Every sample was assayed in triplicate, and each experiment was performed with mononuclear cells from four donors. Cell culture supernatants were harvested and stored at −20°C until cytokines were analyzed.
Concentrations of IL-6, TNFα, IL-10 and IL-12 (p70) in culture supernatants of PBMCs were measured by solid phase sandwich ELISA (enzyme-linked immunosorbent assay) employing commercial kits (Pharmingen, CA, USA) according to the manufacturer's instructions. Cytokine concentrations were obtained from standard curves. Analytical sensitivities were 2.0pg/mL for TNF-α, 2.2pg/mL for IL-6, 4.0pg/mL for Il-12 (p70) and 2.0pg/mL for IL-10. Data were expressed as the mean±standard error for mean (SEM). Results were analyzed by analysis of variance – one-way ANOVA followed by the Dunnett's multiple comparisons test (several parameters against the control group) or the Student's t-test (two parameters) using GRAPHPAD PRISM ver. 4.00 for Windows (GraphPad Software, USA). For toxicity of PEF7121 – challenged mice were compared between groups using log rank test.
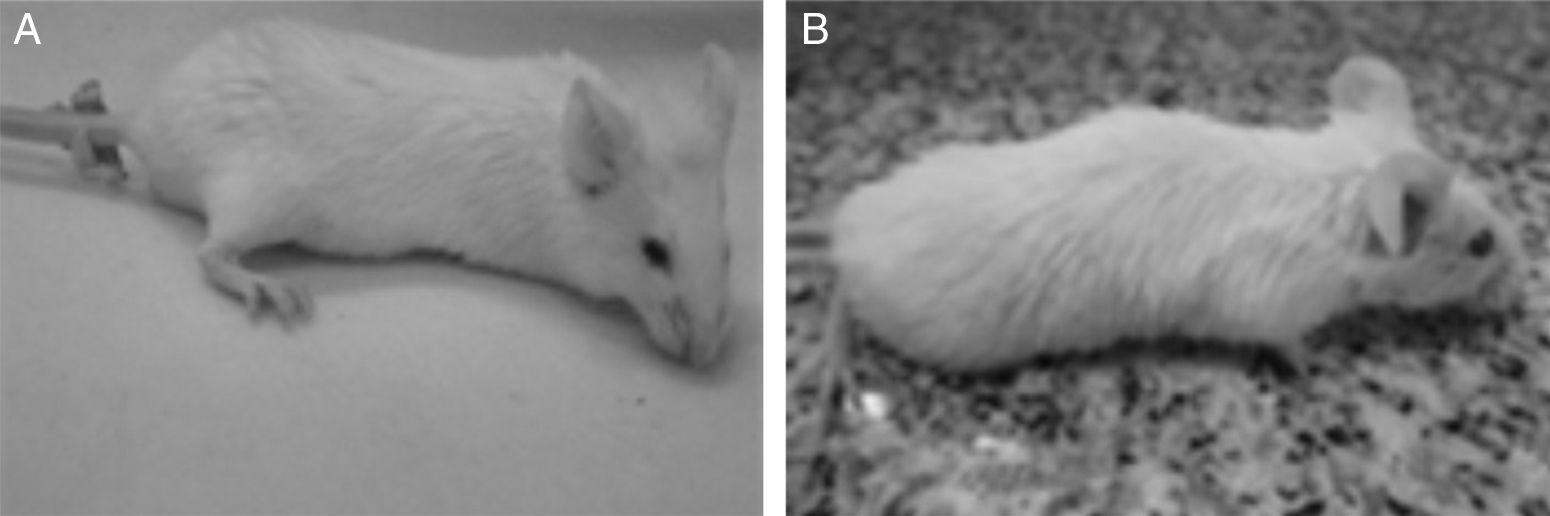
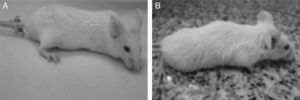
Changes in the appearance and behavior of mice were first evidenced on day 2 post-challenge in the group D and the general health score of these animals was S4. The control and groups A, B and C obtained the highest score throughout the study (S5). At the end of the experiment (day 15 post-challenge) 100% of the animals had survived (Fig. 1).
General Health Score in BALB-C mice injected intraperitoneally with cell wall extract of E. faecalis CECT7121 (PEF7121). (A) 5000μg PEF7121 score 5, bright eyed and alert, a smooth coat with sheen, responds to stimulus, shows interest in environment. (B) 10,000μg PEF7121, score 4, fur slightly ruffled, a loss of sheen of the coat, mouse remains alert and active.
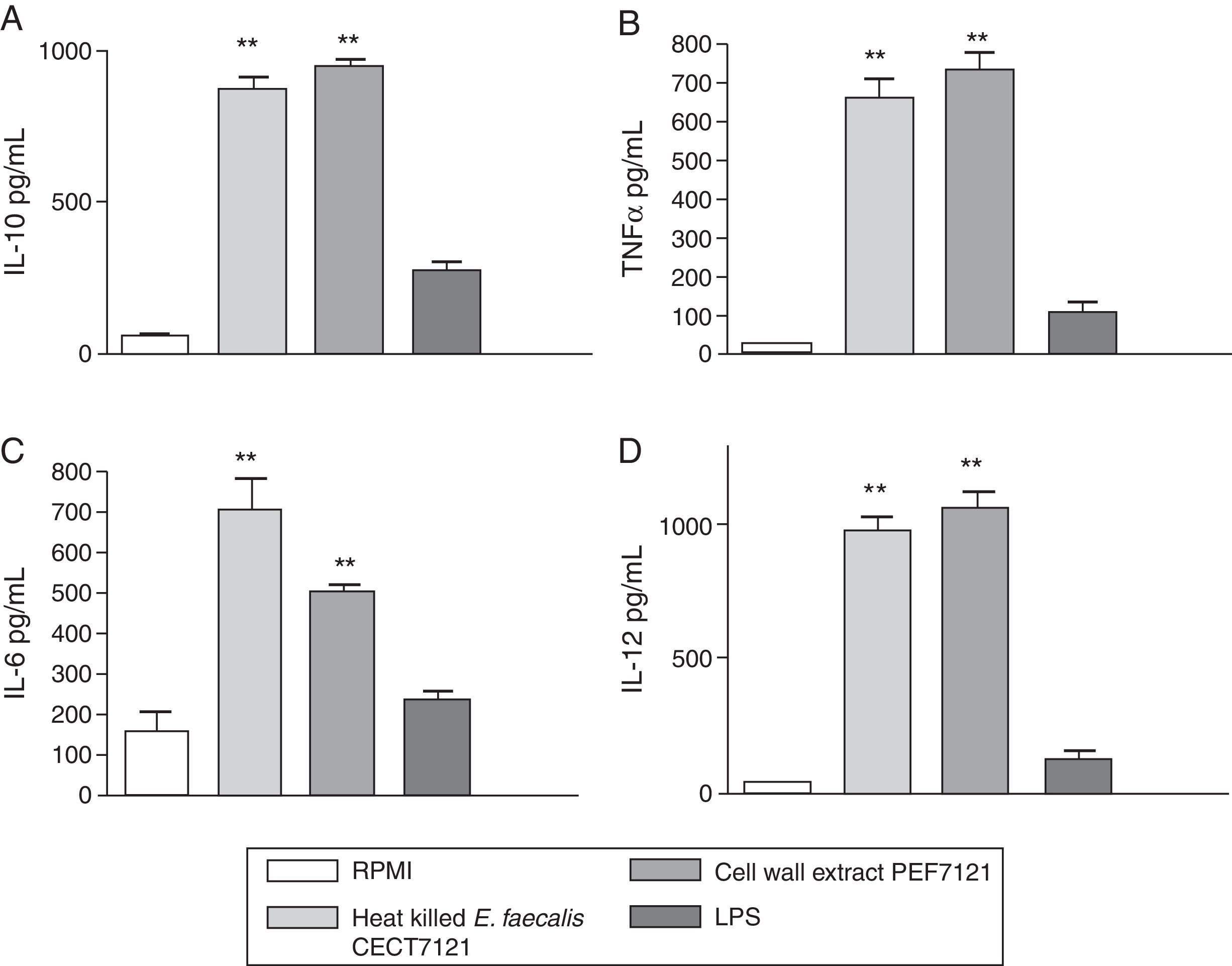
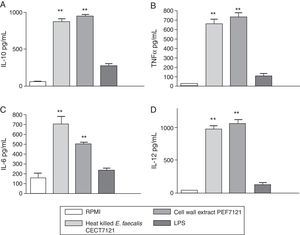
Results of the effects of PEF7121 on cytokine production by PBMCs are shown in Fig. 2.
Cytokine production by peripheral blood mononuclear cells (PBMCs) stimulated with cell wall extract PEF7121. Concentration of IL-10 (A), TNFα (B), IL-6 (C) and IL-12 (D) by PBMCs (area under the curve of mean±SE) after incubation with heat killed E. faecalis CECT7121, cell wall extract PEF7121 and LPS (**p<0.01, ANOVA, Dunnett's post-test). Triplicate measures were determined in four independent assays. IL: interleukin; LPS: lipopolysaccharide; TNF: tumor necrosis factor.
PEF7121 and heat-killed E. faecalis CECT7121 induced the production of significantly higher amounts of both Th1-type cytokines, IL-12, IL-6 and TNF-α and the regulatory cytokine IL-10 compared to the nonstimulated PBMCs (p<0.01).
The mean cytokine production in stimulated PBMCs was as follows: (a) heat-killed E. faecalis CECT7121:868.5pg/mL for IL-10, 655.8pg/mL for TNF-α, 704.0pg/mL for IL-6 and 976.8pg/mL for IL-12; (b) PEF7121:942.6pg/mL for IL-10, 731.6pg/mL for TNF-α, 504.9pg/mL for IL-6 and 1061.3pg/mL for IL-12; (c) LPS: 280.9pg/mL for IL-10, 108.1pg/mL for TNF-α, 236.4pg/mL for IL-6 and 125.7pg/mL for IL-12. There were no significant differences in PBMCs stimulation of IL-10, IL-12 and TNFα between heat-killed cells and PEF7121 (p>0.05), but the IL-6 induction was superior with heat-killed E. faecalis CECT7121 (p<0.05). After 24h PEF7121 induced the release of ILs and TNF-α in significantly higher amounts than those achieved by LPS-induction.
In this study, ability of cell wall extract of the probiotic strain E. faecalis CECT7121 to stimulate production of pro- and anti-inflammatory cytokines using activated human monocyte model was investigated. Effect of heat-killed cells on cytokine production was confirmed by PEF7121, indicating that bacterial cell envelope components are important in determining immunostimulating activities of probiotic bacteria. It was proven that PEF7121 is a potent inducer of TNF-α, IL-6 and IL-12 release from human PBMCs in quantities even higher than those induced with LPS. The significant differential effects detected in IL-6 stimulation between the use of heated cells and cell wall extract as stimulants might have been due to alteration of PBMCs’ receptors during the procedure of sonication. In addition, other important compounds for immune stimulation could have been lost along during the obtainment of PEF7121.
Induction of pro-inflammatory cytokines could indicate that this extract can stimulate nonspecific immune responses. Induction of IL-10 by PEF7121 is expected to participate in the down-regulation of inflammation, since this cytokine can inhibit the functions of macrophages and T cells and promote the development of regulatory T cells. Therefore, it is important to achieve a balance in the profiles of pro- and anti-inflammatory cytokines. Outcomes of this study indicate that PEF7121 would be able to either suppress or stimulate inflammatory responses.
The use of non-viable material of microbial origin has a remarkable advantage over probiotics, since it allows the generation of safer and more stable products. Until now, immunological studies of E. faecalis CECT7121, or its non-viable material such as cell wall extract, have not been performed in a human cell model. For this purpose PBMCs were used, which are easily available and express TLR2 and TLR4 receptors as well as CD14 which have shown to mediate immune response against microbial components such as peptidoglycan.14
In previous studies, it was demonstrated that E. faecalis CECT7121 implants itself and persists in the intestine of BALB/c mice stimulating the innate immune system. It was also proven that peritoneal macrophages from mice intragastrically (IG) pre-treated with E. faecalis CECT7121 had higher capacity for responding to certain microorganisms by secreting higher levels of inflammatory cytokines.9 Finally, it was observed that the IG administration of E. faecalis CECT7121 exerted an adjuvant effect acting as a Th1 modulator in the anti-tetanus and anti-diphtheria immune responses at the systemic level.12
Results of this study further suggest that surface structures of lactic acid bacteria are important in determining immune responses. Inflammatory interleukins’ stimulation patterns shown by PEF7121 are in line with those observed by Medina et al.15 These authors concluded that cell-surface components of Bifidobacterium longum ATCC 15707 and BIF53 strain induced a significant production of both Th1-type cytokines, IL-2 and IFN-γ. Although in discordance with PEF7121, those components have not affected IL-10. Induction of IL-10 was also found by Shida et al.16 using Lactobacillus casei, strain Shirota. In vitro, several species of lactobacilli have shown to trigger the release of IL-6 and TNF-α from PBMCs in amounts comparable to those induced by E. coli.17 Recent studies have suggested that probiotic Lactobacillus strains may have anti-inflammatory activities that directly modulate the host immune system.18Also, previous studies have demonstrated inflammatory cytokines’ induction by cell components of lactic acid bacteria.19,20
According to other authors, bacteria show different immunostimulation profiles. Each probiotic strain has unique and different properties and probiotic effects of a specific strain must not be extrapolated to others.3,19 Comparison between reference strains, human isolates and commercial probiotics has shown that there is a strain-specific pattern of cytokine production, particularly for IL-10 and TNF-α.15 Some of these features are more desirable than others, being necessary for a careful selection of these probiotic strains.
In this study, a role of immnomodulator and promoter of innate defenses is suggested for PEF7121.These findings provide helpful information on immunomodulation activity by cell wall extract in sight of the application of this compound in controlling certain infectious diseases.
Conflicts of interestThe authors declare no conflicts of interest.