In this paper a disseminated persistent Nocardia cyriacigeorgica infection in an immunocompetent patient is described. The patient's long-term treatment, as well as its implications for managing similar cases in the future, is emphasized. Presenting with high fever, multiple nodules, and ulcerative cutaneous lesions of body sites, the patient was treated with various antimicrobials. Under combined therapy, empyema and arthritis, leading to disseminated nocardiosis, were seen. The overall treatment course was 28 months. It can be concluded that the choice of the antibiotics and optimal duration of treatment are uncertain; therefore the treatment of nocardiosis requires expertise.
Herein we describe a case of disseminated Nocardia cyriacigeorgica infection, a recently identified species, in an immunocompetent patient. The infection was most likely acquired from direct inoculation of body surfaces as a result of occupational exposure. Different factors that may have contributed to the long-term treatment of the disease and the subsequent relapse in this patient in spite of in vitro susceptibility of the isolate to all drugs administered are discussed.
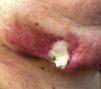
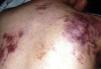
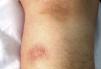
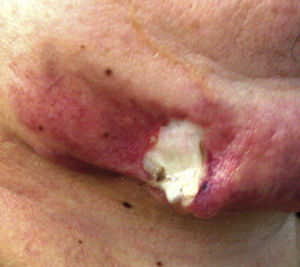
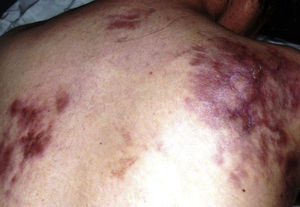
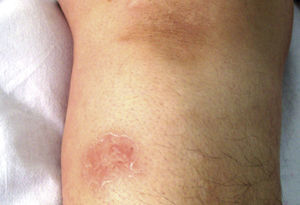
Case reportPatient clinical statusA 45-year-old woman, who has been working as a farmer was hospitalized with symptoms of high fever, multiple nodules, and ulcerative lesions of various body sites. She had no underlying diseases and only had scratches on her hand as a probable indicator of occupational trauma. Previously she had been treated for cellulitis and lymphedema in the ward of internal medicine for two weeks and then moved to the infectious diseases clinic. She presented with fever (38.5°C), multiple cutaneous, erythematous, edematous nodules, and abscesses over the left breast and on the left upper and lower extremities. Similar edematous or ulcerative lesions were later seen under the chin (Fig. 1), on dorso-thorocal, and lombar regions. The lower extremities were edematous. Under therapy some partial resolution of cutaneous lesions were observed. Also, painful new nodules appeared in the following two weeks and some existing nodules continued to expand in size. The nodules and ulcerative lesions varied in size from 2 to 12cm and were tender and erythematous. Remarkable resolution of some cutaneous and subcutaneous lesions (Fig. 2) was seen. Some of the pre-existing nodules have ulcerated and that underwent surgical debridement. Despite in vitro susceptibility of the isolate to all drugs administered, new pyogenic abscesses, empyema of the thorax, and respiratory distress have ensued. The chest radiograph showed left-sided pleural collection. Thoracocentesis and surgical drainage were performed; pleural fluid was obtained for culture. Pleural empyema also occurred on the right side of the lung within two weeks; again surgical drainage was performed. Three months after admission, empyema and skin nodules had fully resolved with scarring. Under suppression maintenance therapy, relapsing fever and cutaneous nodules (Fig. 3) have ensued which were later cleared by an additional two months of treatment. Six months later with combined antimicrobial therapy, the patient was discharged from the hospital on doxycycline maintenance monotherapy. Arthritis of the right knee and ankle, high fever, and new cutaneous lesions developed within a month of hospital discharge and the patient was again hospitalized. The patient presented with high fever and new nodules. A new drug (linezolid) which was not on the market at that time was initiated. After clinical and laboratory response to therapy at the end of one year, some fluctuating cutaneous symptoms had appeared for a period of ten more months. The patient then remained healthy in the following three years of follow-up (Table 1).
Features of disseminated Nocardia cyriacigeorgica infection.
| Symptoms | Laboratory findings | Treatment | Duration of therapy | Clinical outcome |
|---|---|---|---|---|
| High fever, new nodules | ESR 10mm/h, WBC 7070mm–3, Hb 13.8g/L, CRP 9.7mg/dL | Ceftizoxime 6g/d+clindamycin 1800mg/d | 1st⇒14th days | Partial resolution, new lesion (chin) |
| Ulcerative lesion under chin | Gram stain: small rods and coccus | TMP/SXT 10mg/kg/day+Imipenem 2g/day | 14th⇒45th days | New lesions (dorso-thorocal region) bone marrow suppression (WBC 7240mm–3, Hb 6.7g/dL, Ht 22.1, PLT 118,000mm–3)“Breakthrough” |
| Disseminated skin lesions+respiratory distress | Chest radiogram: Pleural empyema Pleural fluid: “Aerobic nocardiform actinomycetesESR 63mm/h, CRP 21.6mg/dL | Imipenem 2g/day+amikacin 1g/day+doxycycline 200mg/daySurgical: drainage and debridement | 45th⇒90th days | Resolution of cutaneous lesions and empyema |
| Scarred cutaneous lesions | ESR 38mm/h, CRP 0.65mg/dL | Amikacin 1g/day+doxycycline 200mg/day | 3rd⇒4th months | Relapse |
| High fever, new nodules | ESR 85mm/h, CRP 5.2mg/dL | Imipenem 2g/day+amikacin 1g/day+vancomycine 2g/day | 4th⇒6th months | Suppression or cureDischarge from hospital |
| None | ESR 45mm/h, CRP 0.66mg/dL | Doxycycline 200mg/day | 6th⇒7th months | Re-activation |
| Arthritis (right knee and ankle) | ESR 95mm/h,CRP 21mg/dL | Ceftriaxone 2g/day+amikacin 1g/day+doxycycline 200mg/day | 7th⇒9th months | HospitalizationNo response, new symptoms |
| New nodules formation (subclavian catheter region) | ESR 104mm/h, WBC 8800mm–3, Hb 12.7g/L, CRP 14.7mg/dL | Linezolid 1200mg/day+doxycycline 200mg/day | 9th⇒12th months | Suppression or cure+adverse effects due to linezolid |
| Peripheric neuropathy, severe malaise | ESR 42mm/h,CRP 1.06mg/dL | TMP/SXT 5mg/kg/day+doxycycline 200mg/day+B6 vitamin | 12th⇒15th months | Resolution discharge from hospital (13th month) |
| Fluctuating mild cutaneous symptoms | ESR 26mm/h, Hb g/L, Ht 39.8, CRP 0.73mg/dL | TMP/SXT 5mg/kg/day+doxycycline 200mg/day | 15th⇒22nd months | Resolution and cure |
| None | ESR 10mm/h,WBC 8080mm–3, Hb 14.2g/L, Ht 43.1CRP 0.3mg/dL | TMP/SXT 5mg/kg/day+doxycycline 200mg/day | 22nd⇒28th months | Well-being |
Erythrocyte sedimentation rate (EST) was 10mm/h, blood count was as follows: leukocyte 7070mm–3, Hb 9.1g/L, Ht 32.5, platelet 419,000mm–3. CRP was 9.7mg/dL. All biochemical tests except moderate hypoproteinemia (albumin 3mg/dL, globulin 1.9mg/dL) were within normal ranges. Chest radiograph and urinalysis were also normal. Gram-stained preparations of pus from ulcerative lesions showed mostly Gram-positive small rods and coccoid fragments within leukocytes. Ziehl–Nielsen stained preparations were negative. Bacteriological culture showed no growth. Later, abscesses material and pleural fluid cultured on Myco/F-Lytic BACTEC liquid medium (brain–heart and 7H9 Middlebrook) and Loewenstein–Jenseen medium grew presumptive Nocardia species in a pure culture within one week. Branching rods were seen in Ziehl–Nielsen with 1% sulfuric acid (modified Kinyoun technique) stained preparations, a typical feature of nocardiae.1–4 The microorganism was confirmed as N. cyriacigeorgica at the reference laboratory in France. The strain was reported as sensitive to amikacin, gentamicin, cefotaxime, ceftriaxone, cefepime, imipenem, vancomycin, trimethoprim/sulphamethoxazole (TMP/SXT), minocycline, doxycycline, and linezolid. Disk susceptibility test was performed according to Boiron and Provost.5 Empyema of the thorax was diagnosed by chest radiograph and chest computed tomography (CT). Further tests did not reveal any evidence of an underlying immunocompromised state.
Treatment and clinical outcomeCeftizoxime and clindamycin combination (two weeks) were switched to TMP/SXT plus imipenem (one month) for treating one of those fastidious microorganisms including Rhodococcus, Nocardia, Actinomyces, Peptococcus, etc., which was suspected as a causative agent based on the Gram smear. Surgical debridement of the dorso-thorocal lesions and surgical drainage of the left-sided and then the right-sided empyema fluid were also implemented in addition to medical therapy. Bone marrow suppression due to TMP/SXT with a 10mg/kg/day trimethoprim dose had developed. The targeted antibiotic therapy to the isolated Nocardia was then a combination of imipenem, amikacin, and doxycycline for 45 days. The response to this regimen was good. As maintenance therapy amikacin plus doxycycline (one month) was given but this regimen had failed and the disease symptoms reactivated with high fever and new nodules’ formation. Therapy was discontinued after six months and the patient discharged from the hospital. Maintenance therapy with doxycycline monotherapy was prescribed. Approximately a month later the disease recurred with symptoms of arthritis and followed by high fever and cutaneous nodules. Neither resolution nor progression of symptoms was observed by combining ceftriaxone, amikacin, and doxycycline for two additional months. After nine months from initiation of disease symptoms linezolid, a drug not on the market at that time, in combination with doxycycine was started. Clinical cure of the disease was obtained after linezolid therapy for three months but eradication of the organism could not be achieved due to cessation of linezolid, because of significant adverse effects (ESR and CRP levels decreased to 26mm/h from 104mm/h and to 0.73mg/dL from 14.7mg/dL, respectively). Mild relapsing episodes of cutaneous lesions were treated with TMP/SXT (5mg/kg/day) and doxycycline for ten more months till no remission and the same antimicrobial combination was further lengthened for six more months after resolution of all disease symptoms with no side effects. The overall treatment course was 28-month long. The patient remained healthy and had no signs of relapse after being followed-up for additional three years without therapy.
DefinitionsDisseminated nocardiosis was defined as nocardia infection in two or more non-contiguous sites. Breakthrough nocardiosis was deemed when a recurrent nocardial infection occurred in a patient receiving systemic antibacterials with known in vitro activity against Nocardia spp. Relapse or reactivation of the disease was noted when an initial improvement was followed by reappearance of clinical symptoms and laboratory findings.6
DiscussionThe literature survey of post-treatment follow-up of Nocardia infections is often too brief or unknown, making the ultimate success of therapy uncertain.7 Members of the N. asteroides complex are more frequently involved in pulmonary infections. Recently, several new species have been described in this complex. The present report is a case of disseminated persistent infection in an immunocompetent patient with primary cutaneous involvement, empyema, and arthritis due to N. cyriacigeorgica, which was formerly part of the N. asteroides complex. Previous cases of N. cyriacigeorgica infections have been reported in immunocompromised patients.8,9
The experience with nocardiosis suggests that when the infection is disseminated, the clinical response is slow.10 Therefore successful therapy requires combination of antimicrobial drugs and appropriate surgical drainage. The optimal antimicrobial therapy depends on the severity and localization of the infection, the species of Nocardia, host immune status, potential drug interactions, toxicity associated with antibiotic usage, and duration of illness prior to diagnosis.1,2,11
Imipenem and amikacin seemed to be the most effective agents, and in vitro synergism has been demonstrated between imipenem and TMP/SXT, imipenem and cefotaxime, amikacin and TMP/SXT.1,2,12,13 Although synergy has been reported in the literature, Kanemitsu et al.14 described that synergy was present in 83% of 23 N. asteroides strains treated with amikacin and TMP/SXT, in 26% of 15 strains treated with amikacin plus ceftriaxone, and in 5% of 26 tests conducted with amikacin and imipenem, thus showing that synergic effect of antimicrobial combinations were not observed in all cases. In the present case, combination therapy with imipenem, amikacin, and doxycycline did not improve disease outcome unless vancomycin was added.
New antimicrobial agents are needed. The relatively high incidence of adverse events, such as diffuse rash and myelosuppression occurrence during sulfonamide therapy, has been reported. Besides, there is lack of alternative highly active oral agents.1,2,14 Linezolid is the first antimicrobial to be active against all clinically significant species of the genus Nocardia.14,15 It has been reported to be effective in treatment, especially in disseminated disease. Because of its activity and availability as an oral agent and the current limitations of the sulfonamides, linezolid has the potential to be the primary drug of choice for treating Nocardia disease.16 Treatment related anemia and peripheral neuropathy have been reported,16 which resolved when linezolid therapy is discontinued, as observed in the present case.
Remissions and exacerbations lasting for days or weeks are characteristic of the disease.11,17,18 The disease may spread hematogenously leading to long-term persistent nocardiosis.18 In the absence of consensus on the length of therapy, investigators mostly recommend to prolong medication between 6 and 24 months because of the relapsing nature of the infection.6,19 Further progression of cutaneous disease to empyema and arthritis, “breakthrough nocardiosis” under combined therapy was seen in the present case. Therefore, treatment duration of 28 months was the longest reported period in the literature.
The sequence of different combined antibiotics used, for so long in this patient might have implications for managing similar cases in the future. Antibiotics with intracellular activity may be beneficial in long-term treatment of the disease. As there are no unanimous guidelines on the therapy of nocardia infections, at the moment the optimal duration of treatment is uncertain and requires expertise.
Conflicts of interestThe authors declare no conflicts of interest.
The authors are indebted to Prof. Patrick Boiron from Mycology Laboratory of Claude Bernard University, France, and Prof. Ramazan Inci from the Mycology Laboratory of Ege University School of Medicine, Izmir, Turkey, for their contributions in identifying N. cyriacigeorgica in species level.