There are no reports on hyponatremia and acute kidney injury (AKI) involved in the course of HIV-related toxoplasmic encephalitis (TE). The main objective of this study was to describe the occurrence of hyponatremia and its relationship with AKI and mortality in HIV-related toxoplasmic encephalitis (TE).
MethodsThis was a retrospective cohort study on patients with HIV-related TE. AKI was considered only when the RIFLE (risk, injury, failure, loss, end-stage) criterion was met, after the patient was admitted.
ResultsA total of 92 patients were included, with a mean age of 36±9 years. Hyponatremia at admission was observed in 43 patients (46.7%), with AKI developing in 25 (27.1%) patients during their hospitalization. Sulfadiazine was the treatment of choice in 81% of the cases. Death occurred in 13 cases (14.1%). Low serum sodium level correlated directly with AKI and mortality. Male gender (OR 7.89, 95% CI 1.22-50.90, p = 0.03) and hyponatremia at admission (OR 4.73, 95% CI 1.22-18.30, p = 0.02) were predictors for AKI. Independent risk factors for death were AKI (OR 8.3, 95% CI 1.4-48.2, p < 0.0001) and hyponatremia (OR 9.9, 95% CI 1.2-96.3, p < 0.0001).
ConclusionAKI and hyponatremia are frequent in TE. Hyponatremia on admission is highly associated with AKI and mortality.
Toxoplasmic encephalitis (TE) is the most frequent neurologic disorder in patients with advanced human immunodeficiency virus (HIV) infection.1,2 Low CD4+ T-cell count and lack of prophylaxis with trimethropim-sulfamethoxazole are known risk factors for developing TE.3
Although scarcely studied in TE, alterations in serum sodium regulation constitute a common feature in neurologic disorders.4 Neurologic-associated hyponatremia could be due to the syndrome of inappropriate antidiuretic hormone secretion (SIADH), also known as cerebral salt-wasting syndrome, which is clinically challenging to diagnose with accuracy.5 Hyponatremia per se is associated with a reserved prognosis.6 However, the association with TE is not fully established, with studies suggesting that hyponatremia may be a mere indicator of the severity of illness.7
In the general HIV-infected population, risk factors for developing acute kidney injury (AKI) are well established (hepatitis virus C infection, opportunistic infections, nephrotoxic drugs), as well as their implications on prognosis.8,9 HIV-infected patients with TE are, in theory, at increased risk for developing AKI, mainly because the treatment for TE consists of using a nephrotoxic drug, sulfadiazine.10
Sulfadiazine is a short-acting sulfonamide derivative that undergoes acetylation in the liver to a variable degree (10% to 40%). Sulfadiazine-related nephrotoxicity is through crystalluria due to acidified urine, leading to tubular obstruction.11 Although many published case reports describe sulfadiazine-related crystalluria and its association to AKI, incidence, risk factors, and evolution remain unclear.
Moreover, in some studies, AKI has been independently associated with hyponatremia, with the latter being considered a consequence of the former. This study aimed to analyze hyponatremia in HIV-infected patients with TE, its temporal relationship with AKI, and involved prognostic implications.
MethodsStudy designThis study was retrospective cohort study conducted at a tertiary and reference center for infectious diseases (Hospital São José de Doenças Infecciosas) in the city of Fortaleza, Brazil. HIV-infected patients with TE diagnosis, from March 2002 to November 2007 were included in the study. Patients with end-stage renal disease undergoing maintenance dialysis were excluded. The study protocol was approved by the local Institutional Ethics Committee, protocol number 021/2007, on 20 August 2007. Due to the retrospective nature of the study, the institutional review board waived the participants’ informed consent.
Data collectionThe clinical investigation included a review of all demographic characteristics, clinical signs, symptoms presented by each patient upon hospital admission, and use of antiretroviral drugs. Laboratory data included the assessment of serum urea, creatinine, transaminases (AST, ALT), direct and indirect bilirubin, lactate dehydrogenase (LDH), complete blood count, CD4+ T-cell count, HIV viral load, and urinalysis.
DefinitionsA presumptive diagnosis of TE was made according to the criteria proposed by the Centers for Disease Control and Prevention, USA, which include recent onset of a focal neurologic abnormality consistent with intracranial disease or a reduced level of consciousness; evidence by computed tomography or magnetic resonance imaging of a lesion exerting ‘a mass effect’ or a cranial radiographic image which is enhanced by injecting a contrast medium; and the presence of serum antibodies against Toxoplasma gondii or a successful clinical response to appropriate therapy for toxoplasmosis.
Admission hyponatremia was considered only when the patient presented a serum sodium concentration of less than 135 mEq/L at admission; severe hyponatremia was defined when the patient had a serum sodium concentration of less than 125 mEq/L upon admission.
AKI was defined according to the RIFLE criteria: risk (“R”) when measured maximum serum creatinine (SCr) was 1.5 times greater than the basal SCr; injury (“I”) when the maximum SCr was twice the value of the basal SCr, or failure (“F”) when maximum SCr was three times the value of the basal SCr. According to the RIFLE criteria, if a patient basal SCr is equal or greater than 4mg/dL, only an increment of 0.5mg/dL is sufficient to classify the patient in the “F” category. No patients with loss, (“L”) or end-stage (“E”) were included in the study. The basal SCr was considered to be that obtained at admission. AKI was considered only if the SCr increment occurred after hospital admission. Therefore, irrespective of the patients’ SCr, an improvement after admission was not considered as AKI. Urine output was not integrated in the classification according to the RIFLE criteria due to lack of recorded information in the medical records. The estimated glomerular fitration rate (eGFR) was calculated using the simplified MDRD equation. HIV-associated nephropathy (HIVAN) was not a possible diagnosis in the studied cases, since the patients had no significant proteinuria.
Treatment protocolThe first line therapy for TE was a daily dose of pyrimethanine 75-100mg over the first three days, and a subsequent daily dose of 25-50mg, plus oral sulfadiazine 500-1,000mg four times a day, during four weeks. Additionally, all patients under treatment received a daily dose of folinic acid.
Statistical analysisStatistical analysis was performed using the Statistical Package for Social Sciences (SPSS) 17.0 program. Descriptive statistics were expressed as mean±SD. The primary analysis compared AKI to non-AKI patients, presence of hyponatremia on admission, and indicators of hospital mortality. All variables were tested for a normal distribution using the Kolmogorov-Smirnov test. Student's t-test was used to compare the means of the continuous variables of normally distributed data. All categorical data were tested using the chi-squared test. Data related to the severity of hyponatremia on admission were tested using the analysis of variance (ANOVA) test; Tukey's post hoc test was used for numerical values, and the chi-squared test was used for trends in assessing categorical data. A multiple logistic regression model was constructed, and association measures were calculated (adjusted odds ratio), with a 95% confidence interval. Stepwise backward elimination multivariate analysis was performed to investigate the independent risk factors for developing AKI and for hospital mortality, which included factors presenting a significance level < 0.2% in the univariate analysis (Mann-Whitney's and chi-squared tests). p-values < 0.05 were considered to be statistically significant.
ResultsPatientsA total of 92 patients had a diagnosis of toxoplasmosis during the study period. The gender ratio (men:women) was 3.5:1 and the mean age was 36.2 ± 9.0 years. Before admission, 59 (64%) patients were under highly active antiretroviral therapy (HAART). The main drugs used in HAART were zidovudine and lamivudine (48% each), efavirenz (30.4%), and lopinavir/ritonavir and atazanavir (12% each). Other antiretroviral drugs used in less than 10% of the cases included: tenofovir, stavudine, ritonavir, and abacavir. The mean CD4+ T cell count was 128 ± 82 cells/mm3. Six patients had associated diabetes mellitus, while hypertension was present in four patients. Initially, all patients received a sulfadiazine and pyrimethanine-based therapy. In 15 patients, the treatment was changed to clindamycin. The mean hospital stay was 24 ± 19 days. The complete laboratory data are shown in Table 1.
Comparison of patients with toxoplasmic encephalitis according to the occurrence of hyponatremia and AKI.
| Parameter | Hyponatremia(n = 43) | Non-hyponatremia(n = 49) | p | AKI(n = 25) | Non-AKI(n = 67) | p |
|---|---|---|---|---|---|---|
| Age (years) | 37.5±9.9 | 34.6±8.5 | 0.15 | 36.7±9.7 | 35.9±9.0 | 0.70 |
| Gender | ||||||
| Male | 29 (67%) | 33 (67%) | 0.93 | 19 (76%) | 47 (70%) | 0.72 |
| Female | 11 (33%) | 19 (33%) | 6 (24%) | 20 (29.8%) | ||
| Main symptom | ||||||
| Headache | 19 (44.1%) | 22 (44.8%) | 0.89 | 9 (36%) | 32 (47.7%) | 0.25 |
| Motor deficit | 5 (11.6%) | 7 (14.2%) | 2 (8%) | 10 (14.9%) | ||
| Convulsion | 11 (25.5%) | 11 (22.4%) | 4 (16%) | 20 (29.8%) | ||
| Signs and symptoms | ||||||
| Fever | 14 (32.5%) | 7 (14.2%) | 0.03 | 8 (32%) | 16 (23.8%) | 0.47 |
| Weight loss | 26 (50.4%) | 18 (36.7%) | 0.02 | 17 (68%) | 31 (46.2%) | 0.08 |
| Vomiting | 11 (25.5%) | 11 (22.4%) | 0.74 | 7 (28%) | 16 (23.8%) | 0.74 |
| Diarrhea | 17 (39.5%) | 20 (40.8%) | 0.85 | 11 (44%) | 27 (40.2%) | 0.83 |
| Jaundice | 1 (2.3%) | 1 (2.0%) | 0.93 | 1 (4%) | 1 (1.4%) | 0.47 |
| Laboratory tests | ||||||
| Ur (mg/dL) | 32.0±28.5 | 24.6±11.0 | 0.20 | 37.9±34.6 | 24.7±10.4 | 0.03 |
| Cr (mg/dL) | 0.7±0.4 | 0.8±0.3 | 0.08 | 0.8±0.5 | 0.8±0.3 | 0.70 |
| Hb (g/dL) | 10.4±1.8 | 13.5±14.2 | 0.19 | 13.7±1.8 | 11.1±2.0 | 0.30 |
| White blood count (/mm3) | 5005±3441 | 4984±2040 | 0.97 | 5095±3849 | 5003±2167 | 0.89 |
| Platelets (/mm3) | 229254±3.1 | 187905±1.0 | 0.45 | 255485±3.8 | 181518±1.0 | 0.19 |
| AST (IU/L) | 39.4±19.5 | 37.5±16.3 | 0.73 | 41.7±18.0 | 39.8±20.2 | 0.77 |
| ALT (IU/L) | 40.4±22.7 | 47.7±28.9 | 0.36 | 41.2±22.0 | 47.5±31.3 | 0.52 |
| Albumin (g/dL) | 2.8±0.5 | 3.6±0.3 | 0.01 | 2.9±0.8 | 3.0±0.5 | 0.87 |
| Antiretroviral use | 28 (65.1%) | 26 (53%) | 0.24 | 18 (72%) | 40 (59.7%) | 0.35 |
| AKI | 18 (41.8%) | 7 (14.2%) | 0.002 | 25 (100%) | - | - |
| Hyponatremia | 43 (100%) | - | - | 18 (72%) | 20 (29.8%) | 0.002 |
| Death | 11 (25.5%) | 2 (4.0%) | 0.0001 | 8 (32%) | 3 (4.4%) | 0.0001 |
Ur, urea; Cr, creatinine; Hb, hemoglobin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; AKI, acute kidney injury; Student's t-test and Fisher's exact test. Significant p< 0.05.
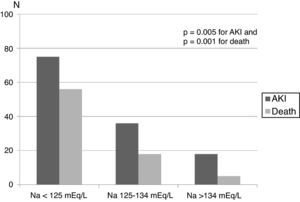
Hyponatremia on admission was verified in 43 (46.7%) patients, and severe hyponatremia (less than 125 mEq/L) in ten cases (10.8%). Serum sodium upon admission was 137.9 ± 2.5 mEq/L in patients with normal range and 129.1 ± 3.9 in hyponatremic patients (range 116-139 mEq/L), p < 0.001. Patients admitted with hyponatremia reported a history of recent weight loss and fever more frequently than normonatremic patients. AKI during hospital stay and mortality was more frequent in hyponatremic patients, especially in patients with severe hyponatremia (Fig. 1). Data showing the comparison among the patients with and without hyponatremia are shown in Table 1.
Mortality rate was higher in patients admitted with hyponatremia, showing a stepwise increment according to the severity of hyponatremia (Fig. 1).
Acute kidney injuryOnly five patients had a GFR of less than 80mL/min/1.73m2 at admission, whose renal function improved during their hospital stay, thus it was possible to diagnose AKI at admission. During hospitalization, 25 patients (27.1%) developed some degree of AKI, considering the SCr on admission as the baseline renal function. The distribution according to RIFLE category was: risk, seven patients; injury, 11; and failure, seven. No patient needed renal substitutive therapy. Mortality rate was 32% among patients with any degree of AKI. Upon multivariate analysis, male gender (OR 7.890, 95% CI 1.223-50.906, p = 0.03) and hyponatremia on admission (OR 4.732, 95% CI 1.223-18.307, p = 0.024) were independently related to AKI developed during hospital stay. A comparison among patients with and without AKI is shown in Table 1.
Factors related to hospital mortalityDeath occurred in 13 cases (14.1%). As demonstrated in Table 2, survivors had comparable clinical and laboratory findings to non-survivors, except but for a higher prevalence of hyponatremia at admission, lower serum sodium (131.3 ± 7.4 vs. 135.2 ± 4.8 mEq/L, p = 0.02) and higher incidence of hospital-acquired AKI. In multivariate analysis, hyponatremia upon admission (OR 4.73, 95% CI 1.22-18.3, p = 0.02) and male gender (OR 7.89, 95% CI 1.22-50.9, p = 0.03) were independent factors related to hospital mortality.
Comparison of survivors and non-survivors patients with toxoplasmic encephalitis.
| Parameter | Survivors(n = 79) | Non-survivors(n = 13) | p |
|---|---|---|---|
| Age (years) | 36.2±9.0 | 36.0±9.6 | 0.95 |
| Length of hospital stay (days) | 22±17 | 32±27 | 0.10 |
| Gender | |||
| Male | 59 (74.7%) | 9 (69.2%) | 0.73 |
| Female | 20 (25.3%) | 4 (30.8%) | |
| Main symptom | |||
| Headache | 37 (46.8%) | 6 (46.1%) | 1.0 |
| Motor deficit | 12 (15.1%) | 0 | |
| Convulsion | 21 (26.5%) | 3 (23%) | |
| Signs and symptoms | |||
| Fever | 19 (24%) | 5 (38.4%) | 0.31 |
| Weight loss | 43 (54.4%) | 6 (46.1%) | 0.76 |
| Vomiting | 21 (26.5%) | 3 (23%) | 1.0 |
| Diarrhea | 36 (45.5%) | 3 (23%) | 0.22 |
| Jaundice | 1 (1.2%) | 1 (7.6%) | 0.26 |
| Laboratory tests | |||
| Ur (mg/dL) | 29±22 | 25±7.2 | 0.64 |
| Cr (mg/dL) | 0.8±0.4 | 0.7±0.2 | 0.43 |
| Hb (g/dL) | 12±10 | 10±1.4 | 0.66 |
| White blood count (/mm3) | 5034±2839 | 5083±2319 | 0.95 |
| Platelets (/mm3) | 214006±2.4 | 168056±1.0 | 0.52 |
| AST (IU/L) | 40±19 | 39±19 | 0.93 |
| ALT (IU/L) | 47±30 | 37±22 | 0.35 |
| Albumin (g/dL) | 3.1±0.7 | 2.9±0.5 | 0.61 |
| Na+ (mEq/L) | 135±4.7 | 131±7.4 | 0.02 |
| K+ (mEq/L) | 4.2±0.7 | 4.7±0.8 | 0.04 |
| Antiretroviral use | 50 (63.2%) | 9 (69.2%) | 0.76 |
| Hyponatremia | 28 (35.4%) | 12 (92.3%) | <0.0001 |
| AKI | 17 (21.5%) | 8 (61.5%) | 0.001 |
Ur, urea; Cr, creatinine; Hb, hemoglobin AST, aspartate aminotransferase; ALT, alanine aminotransferase; Na+, serum sodium; K+, serum potassium; AKI, acute kidney injury; Student's t-test and Fisher's exact test. Significant p< 0.05.
The present study found important associations between hyponatremia, AKI and mortality in patients with TE. A significant proportion of patients presented hyponatremia (more than 40% of cases) at hospital admission. AKI-development and mortality were both associated with the severity of hyponatremia. The data also disclosed an independent relationship between hospital-acquired AKI and mortality in this population. This is the first study to evidence these findings in TE and, moreover, to describe a temporal relationship between hyponatremia and posterior AKI development.
There are reports on the literature describing the involvement of kidneys in animals infected with toxoplasmosis.1,12 One of the few articles on this topic reports a case of nephrotic syndrome in an immunocompetent patient with generalized toxoplasmosis, but with normal renal function, who had complete recovery with supportive therapy.13
Infection with T. gondii rarely complicates to AKI, which is described only as a consequence of sulfadiazine crystalluria.14,15 However, in the present study, a significant association between sulfadiazine use and AKI was not observed. Therefore, it is suggested that other mechanisms may be involved in toxoplasmosis-associated AKI, which need to be elucidated. AKI is a frequent complication in patients with infectious diseases,16,17 which could also occur in T. gondii infection.
The majority of patients in the present cohort were on HAART (64%). The mean CD4+ T-cell count was low. All patients were treated with sulfadiazine. In HIV-infected patients, levels of CD4+ T-cell counts inversely correlate with reactivation of T. gondii infection, and can cause severe histopathological changes.18 Low CD4+ T-cell count could explain the severe complications observed in this study.
Hyponatremia on admission was frequently observed in this study sample (46.7%), and was severe in 10.8% of the cases. The most common causes of hyponatremia among hospitalized patients with toxoplasmosis are SIADH and use of diuretics.19 Hyponatremia is the most common electrolyte disorder in clinical practice, known to be associated with increased mortality in patients with different settings.20,21
In the present study, patients with hyponatremia on admission had a history of recent weight loss and fever more frequently than normonatremic patients. Hyponatremia is seldom described in toxoplasmosis, and can be the presenting feature in some cases.22 As reported in the literature, it occurs in approximately 10% of children with acute central nervous system diseases without renal dysfunction or prior use of diuretics, where complications could be due to SIADH or cerebral salt wasting.23 It is suggested that similar mechanisms are involved in patients with TE. In animals with toxoplasmosis, hyponatremia has also been described in one fatal case.24 However, the precise cause of hyponatremia in toxoplasmosis remains unclear. There are descriptions of SIADH and diabetes insipidus in association with this parasitic infection, which could in part justify the occurrence of this electrolyte abnormality.25
In the present study, hyponatremia occurred before the development of AKI, and it proved to be an important risk factor for AKI. A possible mechanism for this finding could be that hyponatremia triggers release of ADH, leading to increased urinary concentration, which in turn predisposes to sulfadiazine crystal deposition. One of the limitations of the present study is that a majority of the patients did not have urinalysis, which would have allowed studying the urine sediment for crystals.
AKI is an important risk factor for death. In their work, Macedo et al.26 showed that oliguria lasting for more than 12h and of more than three episodes had an increased real risk for mortality. In this sample, mortality in patients presenting any degree of AKI was 32%, which was significantly higher than the overall mortality (14%). Similar mortality rates, varying from 15% to 27%, have been reported.27,28 Nonetheless, currently, no report has associated AKI and hyponatremia to increase risk of dying in TE. In the present study, male gender and admission hyponatremia were independently related to AKI development. Survivors had comparable clinical and laboratory findings to non-survivors, except for higher prevalence of hyponatremia at admission, lower serum sodium, and higher incidence of AKI.
In this study serum sodium levels were significantly associated with mortality, evidencing that hyponatremia per se increased mortality, which is different from what has recently been suggested by others,7 who stated that patients died “with” and not “due to” hyponatremia. Another recent study evidenced that outpatients with chronic heart failure who had hyponatremia presented increased mortality, with this electrolyte disturbance being an independent predictor of all-cause mortality.29 Even small increases in sodium levels are associated with higher risk of death.30 Hyponatremia can be a predictor of death in TE because it indirectly reflects a probable, more severe neurological lesion. As a matter of fact, severe or acute hyponatremia can cause life-threatening cerebral edema.6 Thus, hyponatremia can also be a marker of disease severity, since it can be a sign of the presence of important physiologic derangements.6
In summary, AKI and hyponatremia are common events in toxoplasmic encephalitis. Admission hyponatremia is highly associated with AKI and mortality. As patients presented hyponatremia at admisson and developed AKI during hospitalization, hyponatremia might represent an accessible and reliable marker for an increased risk of AKI and perhaps of death. Increased kidney monitoring, and limited use of nephrotoxic drugs or radiographic contrast agent are suggested for these patients. Hyponatremia, a potentially treatable electrolyte abnormality, is a crucial event in this population. It must be identified and correctly treated, considering that it may per se be associated with cerebral edema, coma, and death in these patients.
Conflict of interestAll the authors declare to have no conflict of interest.
The authors are very grateful to the team of physicians, residents, medical students, and nurses from the São José Hospital of Infectious Diseases for providing technical support to the development of this research and for the exceptional assistance provided to the patients. The present study was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq (Brazilian Research Council). The funding agency had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.