Japanese encephalitis virus (JEV) causes Japanese encephalitis, which is a leading form of viral encephalitis in Asia, with around 50,000 cases and 10,000 deaths per year in children below 15 years of age. The JEV has shown a tendency to extend to other geographic regions. Case fatality averages 30% and a high percentage of the survivors are left with permanent neuropsychiatric sequelae. Currently, there is no cure for JEV, and treatment is mainly supportive. Patients are not infectious, but should avoid further mosquito bites. A number of antiviral agents have been investigated; however, none of these have convincingly been shown to improve the outcome of JEV. In this review, the current knowledge of the epidemiology and the pathogenesis of this deadly disease have been summarized.
Japanese encephalitis (JE) is a common mosquito borne flaviviral encephalitis. It is one of the leading forms of viral encephalitis worldwide, mostly prevalent in eastern and southern Asia, covering a region with a population of over three billion.1 Most infections of JE are asymptomatic, but if clinical illness develops, it causes significant morbidity and mortality. Though underreported, JE causes an estimated 50,000 cases and 15,000 deaths annually.2 JE is a disease of public health importance because of its epidemic potential and high fatality rate. In endemic areas, the highest age-specific attack rates occur in children of 3 to 6 years of age.3,4 Approximately one third of patients die, and half of the survivors suffer severe neuropsychiatric sequelae from the disease.5
Japanese encephalitis virus (JEV) belongs to the family flaviviridae and genus Flavivirus.6 It is a single stranded, positive-sense polarity RNA genome of approximately 11kb in length. The virion of JEV contains three structural proteins – nucleocapsid or core protein (C), non-glycosylated membrane protein (M), and glycosylated envelope protein (E), as well as seven non-structural (NS) proteins – NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS.7 JEV exists in a zoonotic cycle between mosquitoes and pigs and/or water birds. This study reviewed JEV literature from 2000 to 2010, outlining the Indian scenario, clinical depictions, diagnosis, and the prevention of this deadly disease.
Historical perspectiveThe first outbreak of encephalitis attributed to JEV was reported in Japan in 1871. Major epidemics have been reported about every ten years; in 1924, over 6,000 cases were documented in a severe epidemic in Japan.8 In 1935, the prototype Nakayama strain was isolated from the brain of a patient suffering from encephalitis. Thereafter, the virus had been classified with other flaviviruses as a group B arbovirus in the family Togaviridae, Originally the term “type B” encephalitis was used to distinguish this summer epidemic from von Economo's lethargica/sleepy sickness, commonly known as type A encephalitis,5 which occurs in winter with a different clinical presentation. Later on, the designation “type B” was abandoned, and in 1985, JEV was designated under a separate family Flaviviridae, as a member of genus Flavivirus.9 The genus Flavivirus has been named after the prototype yellow fever virus (from the Latin word flavi,), and is comprised of 70 small, enveloped viruses with single stranded positive-sense RNA.5
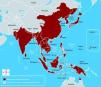
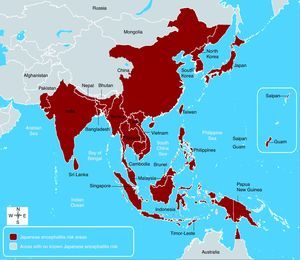
Epidemiological featuresGlobal outlookJapanese encephalitis is one of the most important forms of epidemic and sporadic encephalitis in the tropical regions of Asia, including Japan, China, Taiwan, Korea, Philippines, all of Southeastern Asia, and India; however, related neurotropic viruses are spread across the globe.10 Countries with proven epidemics of JE include India, Pakistan, Nepal, Sri Lanka, Burma, Laos, Vietnam, Malaysia, Singapore, Philippines, Indonesia, China, maritime Siberia, Korea, and Japan.11 In the past 50 years, the geographic areas affected by JEV have expanded (Fig. 1). Epidemic activity in Northern India, Central India, and Nepal has increased since the early 1970s. In the 1990s, the virus continued to spread in Pakistan,12 in the Kathmandu valley of Nepal,13 and also in continental Australia.14 JE is primarily found in Southeast Asian countries. Three epidemiological regions can be distinguished. First, the endemic region composed of Southern India, Southern Vietnam, Southern Thailand, the Philippines, Malaysia, and Indonesia. Secondly, the intermediary subtropical region, which includes Northern India, Nepal, North and Central Burma, Northern Thailand, Northern Vietnam, Southern China, and Bangladesh. Thirdly, the temperate epidemic region, spanning Northern China, Korea, Japan, Taiwan, and the southern extremities of Russia. Transmission is variable, and is coupled with environmental temperature. During winter, mosquitoes are inactive, but huge epidemics can happen during summer and autumn. The geographical area of this disease is showing a trend towards expansion. Postulated explanations are bird migration, certain irrigation projects, animal smuggling, and global warming. Development of rice plantations is theoretically foreseeable in other regions (Pakistan, Afghanistan, Nile Valley, Madagascar, and Oriental Africa), creating a favorable environment for further vector proliferation.15
Problem in IndiaIn India, epidemics of JE are reported from many parts of the country, and it is considered a major pediatric problem. The first recognition of JE based on serological surveys was in 1955, in Tamil Nadu, India.16 A total of approximately 65 cases were reported between 1955 and 1966 in Southern India.17 Subsequent surveys carried out by the National Institute of Virology of Pune indicated that approximately half of the population in Southern India has neutralizing antibodies to the virus. Since 1955, many major outbreaks in different parts of the country have been reported. A major outbreak resulting in a 42.6% fatality rate was reported in the Bankura District of West Bengal in 1973. Subsequently, the disease spread to other states and caused a series of outbreaks in different parts of the country. In 1978, cases were reported from 21 states and union territories.15 In Uttar Pradesh, the first major JE epidemic occurred in Gorakhpur in 1978, with 1,002 cases and 297 deaths reported. Many outbreaks were reported in Gorakhpur after the 1978 JE outbreak, with varying intensity and magnitude. Since 1978 to 2005, this encephalitis has taken more than 10,000 lives in the state.18 The 2005 epidemic surpassed all previous reported outbreaks in the country. In that year, Uttar Pradesh faced a devastating outbreak of JE, mostly confined to Gorakhpur, with 6,061 cases and 1,500 deaths; another outbreak occurred in 2006, with 2,320 cases and 528 deaths. Similarly, JE cases in Uttar Pradesh were confined predominantly to Gorakhpur during 2007, with 3,024 cases and 645 deaths,18 and then onwards till 2007 there have been 103,389 reported cases in India, and 33,729 deaths.19 Approximately 597,542,000 people in India live in JE-endemic regions, and 1,500 to 4,000 cases are reported every year.20 These figures are based on total reported cases; it is possible that many cases are unreported and hence the actual magnitude of the threat of JE may be considerably higher, both in the Indian and in the global context. JE incidence during the past few years is given in Table 1.21 The trend of JE suggests that the problem in Northern India is escalating, and larger epidemics may occur in the future.22
Incidence of Japanese encephalitis in India.
| Sl. No. | Affected States/UTs | 2004 | 2005 | 2006 | 2007 | 2008 | 2009(P) | 2010(P) |
|---|---|---|---|---|---|---|---|---|
| C/D | C/D | C/D | C/D | C/D | C/D | C/D | ||
| 1 | Andhra Pradesh | 7/3 | 34/0 | 11/0 | 22/0 | 6/0 | 14/0 | 132/1 |
| 2 | Assam | 235/64 | 145/52 | 392/119 | 424/133 | 319/99 | 462/92 | 274/59 |
| 3 | Bihar | 85/28 | 192/64 | 21/3 | 336/164 | 203/45 | 325/95 | 19/0 |
| 4 | Delhi | 17/0 | 6/0 | 1/0 | 0/0 | 0/0 | 0/0 | |
| 5 | Goa | 0/0 | 4/0 | 0/0 | 27/0 | 39/0 | 66/3 | 23/0 |
| 6 | Haryana | 37/27 | 46/39 | 2/1 | 32/18 | 13/3 | 12/10 | 0/0 |
| 7 | Karnataka | 181/6 | 122/10 | 73/3 | 32/1 | 3/0 | 246/8 | |
| 8 | Kerala | 9/1 | 1/0 | 3/3 | 2/0 | 2/0 | 3/0 | 19/5 |
| 9 | Maharashtra | 22/0 | 510 | 1/0 | 0/0 | 24/0 | 1/0 | 0/0 |
| 10 | Manipur | 0/0 | 1/0 | 0/0 | 65/0 | 4/0 | 6/0 | 111/5 |
| 11 | Nagaland | 0/0 | 0/0 | 0/0 | 7/0 | 0/0 | 9/2 | 11/6 |
| 12 | Punjab | 0/0 | 1/0 | 0/0 | 0/0 | 0/0 | 0/0 | |
| 13 | Uttrakhand | 0/0 | 0/0 | 0/0 | 0/0 | 12/0 | 0/0 | |
| 14 | Tamil Nadu | 88/9 | 51/11 | 18/1 | 37/0 | 144/0 | 265/8 | 242/3 |
| 15 | Uttar Pradesh | 1030/228 | 6061/1500 | 2320/528 | 3024/645 | 3012/537 | 3073/556 | 1065/172 |
| 16 | West Bengal | 3/1 | 12/6 | 0/0 | 16/2 | 58/0 | 0/0 | |
| Total | 1714/367 | 6727/1682 | 2842/658 | 4024/963 | 3839/684 | 4482/774 | 1896/251 |
C, cases; D, deaths; P, provisionally acute encephalitis syndrome cases.
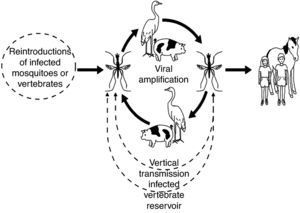
The JEV is transmitted to vertebrates by mosquitoes. Mosquito transmission was suspected during the early 1930s; in 1938, Mitamura et al. reported isolation from Culex tritaeniorynchus.23 The ecology of JEV has come from various studies carried out in Japan by Scherer et al.,24 and JEV ecology has been the subject of several reviews.11,25,26 Many species of Culex mosquitoes can transmit JE. For Southern Asia, Eastern Asia, and Southeastern Asia, the main vector of JE is C. tritaeniorhynchus. For Northern Australia, the main vector is C. annulirostris. However, various other secondary vectors may be important. Indian studies in particular have revealed a number of secondary vectors, including Mansonia indiana, C. pseudovishnui, C. whitmorei, C. gelidus, C. epidesmus, Anopheles subpictus, A. peditaeniatus, and M. uniform.27 The natural cycle of JE virus in Asia involves water birds and Culex mosquitoes. However, unlike many other mosquito-borne diseases, an amplifying host is important in the epidemiology of human JE. In Asia, pigs are considered to be the most important amplifying host, providing a link to humans through their proximity to housing.28 The life cycle of the virus is illustrated in Fig. 2. There are two epidemiological patterns of transmission: an endemic pattern in tropical areas with viral circulation in most months of the year, but with a broad seasonal peak, probably resulting from irrigation practices; and an epidemic pattern in more temperate areas with clear summer seasonality.11,29 Besides mosquitoes, birds also spread the virus to new geographic areas.
Mortality and morbidityJE's mortality rate is approximately 25% to 30%.1,29 Although intensive care support can reduce the mortality rate, patients often suffer significant long-term morbidity. Some effects, such as learning difficulties and behavioral problems, can be subtle and may remain undetected for several years.30,31 50% of those who recover suffer from neurological deficit.32 Over the past 60 years, it has been estimated that JEV has infected more than ten million people, of whom three million died and four million suffered long-term disabilities.29
Recurring patternGenerally, two epidemiological patterns of JE are recognized.29,33 In northern temperate areas (Japan, Taiwan, China, Korea, Northern Vietnam, Northern Thailand, Nepal, and Northern India), large epidemics occur during the summer months, roughly from May to October.34 In southern tropical areas (Southern Vietnam, Southern Thailand, Indonesia, Malaysia, Philippines, Sri Lanka, and Southern India), JE tends to be endemic; cases occur sporadically throughout the year, with a peak after the start of the rainy season (July to September). In India, the state of Karnataka experiences two epidemics each year, with a severe form from April to July and a milder one from September to December along with the rest of India.11
Target populationJE is mostly a disease of children and young adults. Rates of infection in the 3 to 15 year age group are five to ten times higher than in older individuals, because of high background immunity in older individuals. Epidemics in non-endemic regions have affected all age groups, but a bimodal age distribution (young children and elderly) has appeared, indicating an increased risk in elderly people.23 In endemic areas, nearly all residents have sustained infection by young adulthood. The ratio of unapparent to apparent infections is 200:1 to 300:1.35 An excess of cases has been noted in males in many outbreaks; presumably because of increased exposure in areas of rice cultivation.23
Prevalence of the diseaseAlmost half of the human population now lives in countries where the disease is endemic. The annual incidence of the disease is of 30,000 to 50,000 cases,1 and the annual number of deaths reported is 10,000 to 15,000.33,36 The disease can cause irreversible neurological damage.36 A fatality rate of 30% to 50% has been attributed to JE in Southern and Eastern Asia. A large proportion of survivors, 30% to 60% of the cases, suffer from long-term neurological manifestations in the form of convulsions, tremors, paralysis, ataxia, and other such symptoms.28,34 Annual incidence ranged between 1,765 and 3,428 cases and deaths ranged between 466 and 707 in India, according to the National Vector Borne Disease Control Programme of the Ministry of Health and Family Welfare.
Clinical depiction of JEPathogenesisThe incubation period of JEV ranges between six and 16 days. The factors determining who of all the infected develop the disease are unknown, but could include viral factors such as route of entry, titer, and neurovirulence of the inoculum, and host factors such as age, genetic make-up, general health, and pre-existing immunity. After the bite of an infected mosquito, the virus replicates in the skin and is then transported to regional lymph nodes. In most Flavivirus infections including dengue virus, and West Nile virus, Langerhans dendritic cells in the skin are reported to support viral replication.37,38 Next, it amplifies peripherally, causing a transient viremia before invading the central nervous system (CNS).39 During primary viremia, viral particles are seeded in the extraneural tissues. Major extraneural sites of replication include connective tissue, skeletal muscle, myocardium, smooth muscle, lymphoreticular tissues, and endocrine and exocrine glands. From the blood, the virus penetrates into the CNS. The clinical manifestations of many infections are dependent on whether or not the virus gains access to susceptible cells within the CNS. If the infection is limited to extraneural tissues, the signs may be mild or inapparent; however, infection of neural tissues by the same agent leads to encephalitis. Therefore, the mechanism by which the virus penetrates the CNS is of prime importance in understanding the pathogenesis of viral diseases.40,41 How JEV crosses the blood-brain barrier is unknown.5 However, immunohistochemical staining of human postmortem material has shown diffuse infection throughout the brain, indicating a hematogenous route of entry.42,43 Although experimental evidence suggests that replication within endothelial cells may be an important means of crossing the blood-brain barrier in some flaviviruses, for JEV, passive transfer across the endothelial cells appears to be a more likely mechanism.5,40,41 Other factors that compromise the integrity of the blood-brain barrier have also been implicated as risk factors for neuroinvasion. Several studies reported a disproportionate number of fatal cases had neurocysticercosis at necropsy.5,44
Clinical signs and symptomsInfection due to JEV is most often asymptomatic.10 On average, only one in 300 cases produce clinical symptoms. The first signs of infection appear after an incubation period between six and 14 days. It usually starts with a fever above 38° C, chills, muscle pain, and meningitis-type headaches accompanied by vomiting. The initial presentation in children usually begins with gastrointestinal symptoms: nausea, vomiting, and abdominal pains similar to those found in an acute abdominal syndrome.23 These may include confusion, paralysis, Parkinsonian movement disorders, abnormal posturing, seizures, and coma.45 A proportion of patients with JE have an acute flaccid paralysis that is easily mistaken for poliomyelitis,46 but the majority present with a reduced level of consciousness, often heralded by generalized convulsions. Fatality is observed in 20 to 30% of the cases, with signs of acute cerebral edema or severe respiratory distress from pulmonary edema. Recovery usually leaves serious behavioral and neurological sequelae, most notably persistently altered sensorium, extrapyramidal syndrome, epileptic seizures, and severe mental retardation in children. The duration of the coma is associated with repetitive seizures, peduncular damage, or intracranial hypertension, which are considered poor prognostic factors, leading to fatality.47 The course of disease may be divided into four stages. The first is the prodromal stage, which is characterized by an abrupt onset of high fever accompanied by headache, with non-specific symptoms including malaise, anorexia, nausea, and vomiting. The second is acute stage, which includes changes in the level of consciousness ranging from mild clouding to stupors, semi-coma, or coma. Generalized or focal convulsions are common, with neck stiffness and weakness of extremities. In this stage, fatal cases progress rapidly and die. The third is a late stage characterized by defervescence with improved neurologic sequelae in uncomplicated cases. The last stage is the sequelae phase, which includes complete recovery in mild cases, while severe cases also improve, but are left with neurological deficits.
PathologySeveral pathological findings in JE are documented. The main alteration is of the neurological system.8,48 In animal models, nonsuppurative encephalitis could be experimentally induced in piglets inoculated with JE.49 JE predominately affects the thalamus, anterior horn cells of the spinal cord, cerebral cortex, and cerebellum.50 During the acute stage of illness, congestion, edema, and herniation are found in the brain. Microscopic lesions include meningeal inflammation, perivascular lymphocytic cuffing, neuronal degeneration and neuronophagia, and microglial proliferation forming glial nodules. These changes usually occur in gray matter and predominantly affect diencephalic, mesencephalic, and brainstem structures. Immunohistochemical studies of human fatal cases have shown a different topographic distribution of JEV in the brain.43,51 JE virus antigen can be immunohistochemically detected in the cytoplasm of the nerve cells in the cortex of the frontal and temporal lobes, and in the gray matter of the thalamus and midbrain.49 At necropsy, CNS findings in JE reflect the inflammatory response to widespread neuronal infection with a virus.5,8,13,43 The leptomeninges are normal or hazy. The brain parenchyma is congested with focal petechiae or hemorrhage in the grey matter. Blotchy necrolytic zones are seen when survival is prolonged beyond seven days. The white matter usually appears normal. In some patients, the grey matter of the spinal cord is confluent discolored, resembling that of poliomyelitis.5,52 In humans, a characteristic involvement of bilateral thalami can be seen by diffusion-weighted imaging. Magnetic resonance imaging lesions can also be detected in basal ganglia, midbrain, Pons, cerebellum, cerebral cortex, and subcortical white matter. After recovery from acute encephalitic illness, these cases usually manifest clinically with typical Parkinsonian features.53 In addition to brain lesion, the involvement of anterior horn cells can be found as previously mentioned.54,55 The distribution of cell types does not vary between the first and the last day of hospitalization, is similar in fatal and nonfatal cases, and is unaffected by administration of steroids.56
DiagnosisPatients with JE present vivid signs of acute encephalitic syndrome. There are many possible causes of acute encephalitic syndrome; thus, laboratory confirmation is essential for the accurate diagnosis of JE, which is not a simple process due to the very low viremia.1 Diagnosis of JE can be made by virus isolation in cell/tissue culture, antigen detection, and antibody detection.
CultureJapanese encephalitis virus can be isolated by intracerebral inoculation of clinical specimens in the suckling mouse brain. Various cell cultures that have been used more recently include primary chick, duck embryo cells, and lines of Vero, LLCMK2, C6/36, PK, and AP61 cells. The virus can be isolated from the blood of patients in the preneuroinvasive and neuroinvasive phases of the illness, usually not later than six or seven days after the onset of symptoms.50,57
Antigen detectionVarious studies have proved the efficacy of antigen detection in CSF using reverse passive hemagglutination,58 immunofluorescence,59 and staphylococcal coagglutination tests using polyclonal or monoclonal antibodies60 in rapid diagnosis of JE. Modified techniques, such as M-IGSS, have been successfully used in the detection of antigen in mononuclear cells of peripheral blood and CSF of patients.61 Immunohistochemistry has been used to identify viral antigens in the CNS. Histopathology examination is also very helpful for clinical correlation and diagnosis of JEV.
Antibody detectionIgM capture enzyme-linked immunosorbent assay (ELISA) has been the most widely used diagnostic methods for JEV antibody detection.62 At present, much advancement has been achieved with methods for the early detection of JEV, such as the dipstick method63 and JEVCheX.64
PCR diagnosisReal-time polymerase chain reaction (PCR) assays provide sensitivity and specificity equivalent to that of conventional PCR combined with Southern blot analysis, and since amplification and detection steps are performed in the same closed vessel, the risk of releasing amplified nucleic acids into the environment is negligible. In general, both PCR and amplified product detection are completed within an hour or less, which is considerably faster than conventional PCR detection methods. By reverse transcriptase PCR, the viral genome can be amplified directly from tissue or blood.15,65 A novel nested reverse transcription-polymerase chain reaction (RT-PCR)-based kit is described for detecting JEV, in which all reagents are lyophilized in reaction tubes and control RNA is included in each reaction to monitor false negative results.66
Another study described and evaluated a reverse transcription loop-mediated isothermal amplification (RT-LAMP) assay for detecting JEV. The sensitivity of the JEV RT-LAMP assay was in concordance with that real-time RT-PCR, and it was more sensitive than that of conventional RT-PCR. The JEV RT-LAMP was highly specific; no cross-reactivity was found with dengue-2 virus, rabies virus, norovirus, astrovirus, and human enterovirus 71. The JEV RT-LAMP assay was simpler and less time-consuming compared to the conventional RT-PCR and real-time RT-PCR. The results suggest that the RT-LAMP assay can be applied as a practical molecular diagnostic tool for JEV infection and surveillance.67
TreatmentThere is no cure for JE and treatment is mainly supportive. Patients are not infectious, but should avoid further mosquito bites.31 A number of antiviral agents have been investigated, including INF alfa-2a68 and diethyldithiocarbamate (a low molecular weight dithiol).69 However, none of these have convincingly been shown to improve the outcome of JE. Effective supportive management has been shown to improve the outcome.50 The standard management of viral encephalitis should be used.70 Mannitol might be used to reduce intracranial pressure. A significant research on minocycline as an anti-JEV drug is an in vivo study that showed that minocycline reduces neuronal apoptosis, microglial activation, active caspase activity, proinflammatory mediators, and viral titer markedly on the ninth day after infection.71 Another compound that has shown inhibition of JEV replication completely in vitro is an N-methyl isatin-b thiosemicarbazone derivative.72 Supportive nursing care and prevention of infection during hospitalization are important. Close monitoring is necessary for the physiological disturbances during hospitalization and for sequelae after discharge.
Prevention and controlThe prevention of JE is based largely on two interventions; mosquito control, and by an immunization system.
Vector controlVector control is important in primary prevention. To control the vector population, classical methods such as insecticide and bed nets are widely applied in endemic areas.50 Thermal fogging with ultra low volume insecticides such as pyrethrum or malathion has been recommended for the prevention of local transmission during epidemics, particularly in peri-urban areas with marshes. However, the vastness of breeding areas makes larvicidal measures currently impracticable. Effective measures undertaken in some countries to prevent or inhibit larval development include novel water management and irrigation practices such as periodic lowering of the water level, intermittent irrigation, and constant flow systems. Vector control alone cannot be relied upon to prevent JE since it is almost impossible to control mosquito density in the rural areas, which are the worst affected due to poor socioeconomic conditions. Thus, JE control through vector control methods is limited by the sustainability and cost effectiveness of the program.73
ImmunizationTo prevent JE, it is necessary to implement a large-scale immunization of the susceptible human population. Vaccination provides active immunity against JEV. There are several groups of vaccines which are currently in use: purified, formalin-inactivated mouse-brain derived, cell-culture derived inactivated, and cell-culture derived live attenuated.15 Formalin-inactivated vaccines have been safe and effective against JEV for at least 30 years.74 Of these, the most widely produced and internationally distributed is the mouse-brain derived inactivated vaccine. The efficacy and the strain from which these are produced are given in Table 2.
Purified, formalin-inactivated mouse-brain-derived JE vaccineMouse-brain derived inactivated vaccines are based on the Nakayama and Beijing-1 strains (seroconversion rate 80% to 90%). This is the only vaccine against JE approved by the World Health Organization. The vaccine produced from the original Nakayama strain is manufactured in Japan, and was licensed in 1954. It is available internationally under the Biken label.11,23 This vaccine is also independently produced in China, India, Thailand, and Taiwan. The Central Research Institute in Kasauli is the manufacturer in India. It is available in lyophilized form, in which gelatin and sodium glutamate are used as stabilizers, and thimerosal is used as a preservative.75
The primary vaccination is done between the ages of 1 and 3 at doses of 0.5mL to 1mL (0.25 to 0.5 with children under age 3) subcutaneously. The dose regimen consists of one injection on days zero, seven, and 30 with a booster after one year and thereafter every three years until age 10. The protective efficacy is above 90%.15 Due to its high production cost, lack of long-term immunity, and adverse allergic reactions, this vaccine is not practical to be administered in poor rural areas, where it is urgently needed. These difficulties have led to the development of improved vaccines.
Inactivated hamster kidney cell-culture-derived JE vaccineThis vaccine is based on the Beijing-3 strain of JEV.15 In China, an inactivated vaccine produced in primary hamster kidney (PHK) cell culture was developed and has been in use since 1967. It has relatively fewer side effects and is easy to manufacture. In an extensive randomized field trial in China, its efficacy was found to range between 76% and 90%.34 In the last decade, a Vero cell-culture based inactivated vaccine using various local JEV isolates has also been developed and is undergoing clinical trials. A Vero-cell culture derived formalin-inactivated vaccine is being developed using an attenuated SA14-14-2 strain, and it has induced high titers of neutralizing antibodies in mice after two injections.76 Recently, Vero-cell culture derived formaldehyde inactivated JE vaccine using P20778 (Indian isolate) has been developed, and has generated high titers of anti-JEV antibodies in mice; sera from immunized mice neutralized different JEV strains with varying efficacies.77
Cell-culture derived live attenuated JE vaccineThe ChimeriVax-JE has been available since 2001.78 Live attenuated vaccine appears to offer great prospects for future vaccine development, since less virus is needed to trigger a satisfactory immune response, which makes the vaccine cheaper, and fewer doses are required, which makes it easy to administer.5
Vaccine based on the SA14-14-2 strainThis is an attenuated and genetically stable strain that in large-scale case–control studies in China has shown 95% protection after two doses with an interval of one year.15 In the 1980s, China developed this live attenuated vaccine named SA 14-14-2 by passaging the SA14 strain of JEV in PHK cells. Six amino acid changes in E protein and three in NS genes were associated with the attenuation.79 Recently, another case–control study in Nepal showed that a single dose of this vaccine induced an efficacy of 98%.80 A study on the long-term efficacy (over five or ten years) is needed to know if the single dose is sufficient or if boosters are necessary for long-term immunization of the targeted population.
Adverse reactionsThere are several side effects of JE vaccination. Local side effects include tenderness, redness, and swelling. Sometimes systematic adverse reactions are also noted after vaccination, such as headache, myalgia, abdominal pain, or skin rash.15 Occasionally local hypersensitivity reactions (erythema or edema at the injection site) can be observed in some children. Other reactions, such as generalized urticaria, facial angioedema, and respiratory distress have been reported in a few people from non-endemic zones after vaccination.15 Some recipients of the vaccine had, very rarely, major neurological side effects (1 to 2.3 per million recipients: encephalitis, seizures, and peripheral neuropathy).10
Other JE vaccines under developmentSeveral vaccines are still in various stages of development. These include: recombinant protein based vaccines, recombinant virus based/chimeric vaccine, and DNA vaccines. Second generation recombinant vaccines are in development with the aim of improving immunogenecity and decreasing adverse reactions.50
JE vaccination in IndiaThe JE vaccination campaign was launched during 2006 wherein 11 of the most sensitive districts in Assam, Karnataka and Uttar Pradesh were covered. Altogether, 86 JE endemic districts in the states of Assam, Andhra Pradesh, Bihar, Haryana, Goa, Karnataka, Kerala, Maharashtra, Tamil Nadu, Uttar Pradesh, and West Bengal have been covered. Re-orientation training course on AES/JE case management is a continuing process. Such orientating training courses were carried out in Andhra Pradesh, Assam, Haryana, Karnataka, Tamil Nadu, Uttar Pradesh, and West Bengal during 2008 and 2009.18
ConclusionJapanese encephalitis is a public health problem, not only for Asia but for the entire world. However, JE is rising throughout Asia, because epidemics are typically noticed only after outbreaks, and because the disease may go largely unobserved in endemic regions. Environmental and ecological factors are responsible for the spread of JEV. There is no specific treatment for JE; only prevention can control the disease. Control may be possible only after developing a strong surveillance system together with a high-quality immunization program. Implementation of a vaccination program for young children, as well as modified agricultural practices, pig vaccination, rigorous monitoring, vector control, and improved living standards can reduce the number of JE cases.
Conflict of interestAll authors declare to have no conflict of interest.
The author Sarika Tiwari is thankful to the Indian Council of Medical Research, New Delhi for providing financial assistance.