The plague, which is an infectious disease caused by Yersinia pestis, still threatens many populations in several countries. The worldwide increase in human plague cases and the potential use of the bacteria as a biological weapon reinforce the need to study the immunity that is induced by potential vaccine candidates. To determine the immunogenicity of antigenic preparations based on the F1 protein and the total extract from Y. pestis, we assessed the role of these antigens in inducing an immune response.
MethodsThe immunogenicity of antigenic preparations based on the Y. pestis (YP) total extract and the Y. pestis fraction 1 capsular antigen protein (F1) was determined in Swiss-Webster mice immunized with 40μg or 20μg for each preparation. Immunophenotyping was performed by flow cytometry.
ResultsAnimals immunized with the YP total extract did not elicit detectable anti-F1 antibodies (Ab) in the hemaglutination/inhibition (HA/HI) test. Animals immunized with 40μg or 20μg of the F1 protein produced anti-F1 Abs, with titres ranging from 1/16 to 1/8132. The average of CD3+–CD4+ and CD3+–CD8+ T cells did not differ significantly between the groups. Neither YP total extract nor F1 protein induced a significant expression of IFN-γ and IL-10 in CD4+ T lymphocytes. In addition, F1 failed to induce IFN-γ expression in CD8+ T cells, unlike the YP total extract.
ConclusionThe results showed that F1 protein is not an immunogenic T cell antigen, although the YP total extract (40μg dose) favoured CD8+ T cell-mediated cellular immunity.
The plague, which is caused by Yersinia pestis, is essentially a rodent-flea-transmitted disease that affects man and other mammalian species.1 Due to the possibility of using Y. pestis for bioterrorism purposes and the high mortality rate of the disease,2 there is a crucial need to study the immunity induced by potential vaccine candidates for future use as immunoprophylaxis. In recent years, effort has focused on developing a subunit vaccine based on virulence factors of this bacterium.3
Y. pestis F1 capsular antigen protein (Fraction 1 or F1) has been evaluated in several immunization studies that used experimental animals.4–6 Furthermore, vaccines that are formulated with whole cells may contribute to the induction of an effective immune response.7 The mechanism of protection conferred by these preparations has not been fully elucidated, but it has already been shown that an effective vaccine against the plague must induce both humoral immunity and Th1 type cellular response.8
Aiming to determine the immunogenicity of the antigenic preparations based on the total extract from Y. pestis and the F1 protein, we assessed the role of these antigens in inducing the production of antibodies, determining the phenotype of splenic T-lymphocytes and stimulating the production of IFN-γ and IL-10 by subpopulations of CD4+ and CD8+ T cells.
Materials and methodsAnimalsFemale, 6–8-week-old, Swiss-Webster mice (20–24g) were obtained from the Universidade Federal de Minas Gerais (UFMG) facilities. Four animals per cage were maintained with temperatures at 21–24°C, a 12h light/dark cycle, and fed with pelletized food and water ad libitum. Due to the policy of reducing the number of animals used in research protocols, only the minimum number of animals per group was utilized.
Preparation of whole cell extract and Y. pestis F1 antigenY. pestis strain CYP 0579 from the culture collection Fiocruz-CYP was reactivated by inoculation in brain heart infusion broth media (Difco, USA) and incubated overnight at 37°C. The presence of the genes caf1, lcr, pla, and irp2, which are prominent pathogenicity markers,9 was determined by M-PCR according to Leal and Almeida.10 The culture was inactivated by the addition of 2% formaldehyde (Sigma–Aldrich, USA), incubated overnight at room temperature, 23–25°C, and plated on blood agar base (Difco, USA) to confirm bacterial death. The F1 antigen was extracted from the Y. pestis strain A1122 as previously described.11
The Y. pestis formaldehyde-killed suspension (YP) was fragmented by sonication for 90s (2 cycles of 30Hz and 2 cycles of 60Hz intercalated with an ice bath). The YP total extract and F1 protein were subjected to gamma radiation. The protein concentration of the preparations was determined by the Lowry method12 and the products were suspended in PBS at pH 7.2–7.4.
ImmunizationFour groups of four female Swiss-Webster mice were immunized with 40μg or 20μg of the YP total extract and the Y. pestis F1 protein suspensions in PBS plus 25% (v/v) aluminium hydroxide adjuvant, administered in two doses with a 21-day interval. Each animal was injected intramuscularly in the posterior thigh with a total volume of 0.1mL. The control group received the same volume of aluminium hydroxide adjuvant in PBS. After primary immunization, on day 42, the mice received a booster intravenous injection at the base of the tail with 4μg or 2μg doses of the antigens without adjuvant.13 On day 45 after primary immunization, mice were euthanized by anaesthetic overdose. Blood samples and spleens of the immunized and control mice were collected.
Hemaglutination/inhibition test (HA/HI)Sera were collected from immunized animals throughout the study and assayed for the presence of anti-F1 Abs by the HA/HI test.11 The test was considered positive when the HA end-point titre was depressed by three or more dilutions in the HI test. A titre of 1/16 was considered positive.
Preparation of spleen cell suspensionsSingle cell suspensions were prepared from each 2/3 spleen in RPMI 1640 (Gibco, Germany). Each spleen was aseptically collected, and the mononuclear cells were filtered through sterile nylon. The cells were washed in RPMI 1640 and spun down at 440×g for 10min at 18°C. The erythrocytes were lysed with an ammonium chloride solution, and the cells were washed three times in RPMI 1640 and resuspended into RPMI 1640 supplemented with 5% heat-inactivated foetal calf serum (Cultilab, SP, Brazil), penicillin (100UI/mL) and streptomycin (50pg/mL; Sigma–Aldrich, USA).
Spleen cell culture and flow cytometry analysisMononuclear cells were isolated from the spleens of immunized and control mice by Ficoll-Hypaque (Sigma–Aldrich, USA) density gradient centrifugation. After counting the viable lymphocytes, suspensions were prepared containing 1×106splenic cells/mL in RPMI supplemented with 5% foetal bovine serum, 2mM glutamine, 50UI penicillin, and 0.05mg streptomycin (Sigma–Aldrich, USA). Cell suspensions were used for the phenotypic analysis of lymphocytes and for intracellular cytokine analysis.
Cell surface marker staining was performed by adding the previously prepared cell suspension (0.1mL) to flow cytometry tubes containing combinations of the following fluorochrome-labelled Abs: CD3-FITC (145-2C11), CD4-PercpCy55 (RM4-5) and CD8-APC (53–6.7) (Becton Dickinson, USA). Samples were vortexed gently and incubated for 30min at room temperature. The fixing solution containing 4% paraformaldehyde was added to the samples, which were refrigerated until flow cytometric analysis. Lymphocytes were specifically analyzed by selective gating based on size and granularity of the cells using a flow cytometer (Forteza, Becton Dickinson, USA). As negative controls, the respective isotype control (BD) labelled with the same fluorochrome was used. Data analysis was performed using the FlowJo software.
For intracellular cytokine analysis, 1×106 spleen cells from each animal were plated in a 24-well plate and stimulated with 10μg/mL of YP total extract or Y. pestis F1 antigen for 72h. Cultures without added Y. pestis proteins served as controls. Golgi Plug (1μg/mL – Becton Dickinson, USA) was added during the last 4h of incubation. Cells were surface stained with anti-CD3-FITC (145-2C11), anti-CD4-PercpCy55 (RM4-5) and anti-CD8-APC (53–6.7) Abs (Becton Dickinson, USA) and then fixed with 4% paraformaldehyde for 10min. After permeabilization, cells were stained with anti-IFN-γ-PeCy7 (XMG1.2) and anti-IL-10-PE (JES5-16E3) or isotype controls (Becton Dickinson, USA) and analyzed using flow cytometry (Forteza, Becton Dickinson, USA).
Statistical analysesThe difference of the data among groups was compared by analysis of variance (ANOVA) using the R software14 and probability values of <0.05 were considered significant. The number of animals per group (N) was determined using the standard deviation, difference to be detected, and significance level15,16 and was confirmed by the website http://www.lee.dante.br/esquisa/amostragem/amostra.html.17
EthicsMice were handled following the Guide for the Care and Use of Laboratory Animals guidelines.18 The experimental protocols were approved by the Ethics Committee on Animal Experimentation (CETEA) of UFMG (Protocol No. n°148/2014).
ResultsY. pestis anti-F1 antibody (Ab) in animals immunized with different immunization protocolsTo characterize the humoral response induced by immunization with different doses of the YP total extract and the Y. pestis F1 protein, anti-F1 Ab titres were determined by the HA/HI test from serum samples of immunized mice that were collected 45 days post-immunization. Anti-F1 Abs were not detected in the two groups immunized with 40μg (Group 1) and 20μg (Group 2) of YP total extract or in the control group, which received aluminium hydroxide adjuvant in PBS. In the animals immunized with the Y. pestis F1 antigen, the anti-F1 Ab titres in HA/HI ranged from 1/16 to ≥1/8132 in the group receiving 40μg F1 (Group 3) and from 1/64 to 1/256 in the group receiving 20μg F1 (Group 4). It should be pointed out that the volume of sera from some mice of groups 2 (1), 4 (2) and control (1) was not enough for the HA/HI tests (Fig. 1).
Yersinia pestis anti-F1 antibody in mice immunized with different immunization protocols. Group 1: 40μg of YP total extract; Group 2: 20μg of YP total extract; Group 3: 40μg of Y. pestis F1 antigen; Group 4: 20μg of Y. pestis F1 antigen; Control: aluminium hydroxide adjuvant. n<4 (groups 2, 4 and control): the volume of sera was insufficient for the HA/HI tests.
Because the response to immunization involves activation at the cellular level, flow cytometric analysis was performed to investigate whether immunization resulted in changes in cellular activation markers or gross changes of cell counts of mice given different preparations of Y. pestis. Therefore, within the lymphocyte population, the changes in the percentage of splenic T cells were evaluated among immunized groups. No significant differences were observed in the percentages of CD3+–CD4+ and CD3+–CD8+ T cells among the four immunized groups (p>0.05). However, a clear predominance of CD4+ T cells was observed in all groups (Fig. 2A and B).
Phenotypic analysis of splenic T cells after immunization. A: CD3+–CD4+ subpopulations. B: CD3+–CD8+ subpopulations. Group 1: 40μg of YP total extract; Group 2: 20μg of YP total extract; Group 3: 40μg of Y. pestis F1 antigen; Group 4: 20μg of Y. pestis F1 antigen; Control: aluminium hydroxide adjuvant.
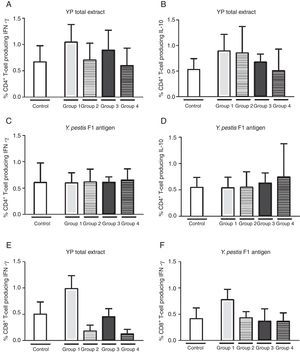
To investigate the response of CD4+ and CD8+ T cells after in vitro stimulation with YP total extract and Y. pestis F1 antigen, spleen mononuclear cells from immunized mice that were collected 45 days post-immunization were cultured and assessed for the production of IFN-γ and IL-10 by flow cytometry. A higher percentage of CD4+ T cells that expressed IFN-γ was observed among the group that received 40μg YP total extract compared to the other groups (Fig. 3A). The levels of CD4+ T cells that expressed IL-10 were higher in the groups that received 40μg and 20μg of YP total extract (Fig. 3B). However, no significant differences were observed in the percentages of CD4+ T cells that produced IFN-γ and IL-10 cytokines after stimulation with YP total extract (p>0.05). The percentage of CD4+ T cells that produced IFN-γ (Fig. 3C) and IL-10 cytokines (Fig. 3D) was lower after stimulation with the Y. pestis F1 antigen than after stimulation with YP total extract. No significant differences were observed in the percentages of CD4+ T cells that produced IFN-γ and IL-10 cytokines among the groups after stimulation with the Y. pestis F1 antigen (p>0.05). The percentage of CD8+ T cells that produced IFN-γ after stimulation with YP total extract was significantly higher in the group that received 40μg YP total extract (Fig. 3E) compared with the other groups (p<0.05). No significant differences were observed in the percentage of CD8+ T cells that expressed IFN-γ (Fig. 3F) among the four groups after stimulation with Y. pestis F1 antigen (p>0.05).
Detection of intracellular cytokines IFN-γ and IL-10 in T cells. (A) CD4+T cells producing IFN-γ and (B) IL-10 cytokines after stimulation with YP total extract; (C) CD4+T cells producing IFN-γ and (D) IL-10 cytokines after stimulation with Y. pestis F1 antigen; (E) CD8+T cells producing IFN-γ after stimulation with YP total extract and (F) after stimulation with Y. pestis F1 antigen. Control: aluminium hydroxide adjuvant; Group 1: 40μg of YP total extract; Group 2: 20μg of YP total extract; Group 3: 40μg of Y. pestis F1 antigen; Group 4: 20μg of Y. pestis F1 antigen.
The present study aimed to investigate the immunogenicity of the antigenic preparations based on the Y. pestis F1 protein and the YP total extract in inducing the production of anti-F1 Abs and in determining the phenotype of splenic T lymphocytes and the stimulation of the production of IFN-γ and IL-10 among the subpopulations of CD4+ and CD8+ T cells in mice. The immunization protocol utilized in our animal experiments was based on a previously established model16 that evaluated the protective efficacy of Y. pestis F1 protein and V subunit vaccines in mice against subcutaneous challenges of the virulent Y. pestis strain. Our results showed that animals immunized with the F1 antigen exhibited a strong humoral response with high titres of anti-F1 Ab in the HA/HI test. Furthermore, it was observed that the animals receiving the lowest (20μg) F1 dose showed titres >1:16, which was the cut-off point in the HA/HI test. These findings were in accordance with another study16 that demonstrated that a dose of 20μg of the F1 antigen induced significantly high IgG titres in mice. The Y. pestis F1 and V subunit vaccines have been the focus of several studies. Although the value of the Y. pestis V antigen is recognized in its protection against non-encapsulated strains (deficient in F1 production), the significant contribution of the F1 protein in the induction of early immune responses is also important. In a study to characterize the immune response of mice after immunization with the F1 protein, quantitative ELISA analysis demonstrated that the levels of anti-F1 IgM antibodies were detectable in serum of animals as early as day 1 post-immunization and that anti-F1 IgG antibodies were detected after three days.19 The ability of anti-F1 Abs to provide effective protection against the bubonic plague has been demonstrated in several studies.19,20 Total protection (100%) against a challenge with a virulent Y. pestis strain was observed in mice immunized with the F1 antigen.19 In another animal model, 80% survival rate was demonstrated with active immunization with the recombinant F1 and V antigen.20 The immunogenicity of recombinant V and F1 proteins may be at maximum because their conformations are similar to those of native proteins.21 Therefore, this finding can explain the high titres of antibodies observed in our study because the antigens used in the immunization experiment were obtained from Y. pestis cultures and not by using recombinant DNA techniques. The use of the aluminium hydroxide adjuvant could also explain the results obtained for the humoral immune response. Furthermore, other studies have reported that subunit vaccines comprising F1 and V antigens in aluminium hydroxide induced a predominant IgG1 response in both mice and humans.16,22 Our results showed that the anti-F1 titres were variable among individuals from the same group, which can be attributed to the mouse lineage used in our study. We chose the experimental model with Swiss-Webster mice, which is a genetically heterogeneous outbred lineage23 that responded in different ways to the same stimulus. The purpose of using that mouse line was to adopt an experimental model that could demonstrate the real efficacy of our putative vaccine because the response to vaccinations may vary among individuals in a population.
Due to the policy of reducing the number of animals in research protocols, only experiments with the minimum number of animals per group were approved by the ethics committee. This would be the best alternative, although the possibility of loss throughout the experiments, e.g., insufficient volume of sera, loss of mononuclear cells when preparing the spleen for cell culture, or microbial contamination of cultivated spleen cell culture, was discussed. In fact, the loss when working with the exact number of animals per group determined an “N” lower than the minimum calculated.
It must be noted that the absence of the humoral immune response to both doses of the YP total extract could indicate that the immunization scheme did not induce detectable levels of anti-F1 antibodies. However, it could be that the YP total extract did not contain a sufficient concentration of F1 protein to induce the humoral immune response in mice.
The role of antibodies in protection against Y. pestis has been verified by several studies.24,25 However, it seems that the humoral immune response induced by subunit vaccines alone may not be sufficient for complete protection against the plague. It was observed that there was 80% protection against a challenge with Y. pestis in mice immunized with the F1 and V antigens; however, 20% of them did not survive, even though high levels of antibodies were found. This finding suggests that protection against Y. pestis is not exclusively mediated by antibodies and depends on the involvement of T cells and their cytokine secretion.20
To determine the changes in the percentage of T cells that respond to antigenic stimulus, we performed immunophenotypic characterization and quantified the mononuclear splenic cells. Our findings indicated a clear predominance of CD4+ T cells in all immunized groups studied. Another study also reported a higher percentage of CD4+ T cells than CD8+ T cells in peripheral blood from Swiss mice. However, our findings showed numbers of CD4+ T cells that were higher than those previously reported,24 which can be justified by the fact that our experiments were performed in spleen T cells. No significant differences were observed in the percentage of T cells among mice from different groups, which suggested that there was no T cell proliferation in response to antigenic stimulation.
Intracellular cytokine analysis showed that the higher dose of YP total extract (40μg) induced higher expression of IFN-γ in CD4+ T cells (group 1). Although no significant differences were observed, this finding may indicate that T cell activation is higher in animals immunized with a higher dose of bacterial total extract. Therefore, increasing the dose of antigen candidates for the vaccine or administering additional doses may represent an alternative to improve the cellular immune response to Y. pestis.
Our study demonstrated that YP total extract induced a lower production of IL-10 in CD4+ T cells; however, the difference was not significant. A higher percentage of CD4+ T cells that expressed IL-10 in groups immunized with the YP total extract (40μg and 20μg) was observed. These results may suggest a Th2 response, which could be confirmed by determining the presence of other cytokines, such as IL-4, IL-5 and IL-6. Given the importance of Th1 and Th2 in protecting against Y. pestis and the possible use of appropriate adjuvants to direct the adaptive immune response, it is highly necessary to find new co-stimulatory molecules that can induce a mixed response based on cytokines and antibody production, which would represent an important innovation in vaccine research field for the plague.
No significant differences were observed in the percentage of T cell activation in response to the F1 antigen. Additionally, our findings revealed that the T cell response induced by the F1 protein was lower than that of the groups immunized with the YP total extract. This finding is consistent with a previous study26 that demonstrated that F1 antigen is unable to induce a strong cellular response in animals immunized with the live attenuated EV76 vaccine, suggesting that the F1 protein may not represent a dominant antigen in T cell activation. Our results also showed that the percentage of CD8+ T cells that expressed IFN-γ was higher in the group that received 40μg YP total extract than in the other groups, which may suggest the role of YP total extract in eliciting a Th1 response mediated by this cellular subpopulation. This finding is highly important because the production of IFN-γ and TNF-α by CD8+ T cells plays a crucial role in activating macrophages and neutrophils, which, in turn, exhibit an increased production of reactive oxygen intermediates that act in the control of intracellular bacteria.27 It was demonstrated that Y. pestis cannot replicate in macrophages of mice activated by IFN-γ.28 Mice whose T cells could not produce IFN-γ and TNF-α did not survive a lethal pulmonary Y. pestis infection.29 Anti-F1 and anti-V Abs protect mice against experimental pneumonic plague, but this protection is highly dependent on IFN-γ and TNF-α production. Therefore, animals that can produce and respond effectively to cytokines seem to protect against Y. pestis infections.30 Our intracellular cytokines analysis exhibited a differentiated response of animals from group 1, which received 40μg YP total extract. These findings may suggest the activation of CD8+ T cells by Y. pestis immunodominant epitopes. To confirm this hypothesis, further studies are needed to identify the proteins in the YP total extract and their contributions to inducing the cellular immune response.
In brief, we showed that the immunization regimen based on the F1 antigen induced a strong humoral immune response, whereas 40μg and 20μg of YP total extract did not induce anti-F1 Ab production in mice. Furthermore, immunization with the YP total extract and the Y. pestis F1 antigen neither promoted CD4+ or CD8+ T cell proliferation nor induced the expression of IFN-γ and IL- 10 by CD4+ T cells; however, it did stimulate the production of IFN-γ by CD8+ T cells. Therefore, we can suggest that Y. pestis F1 protein is not an immunogenic antigen to T cells.
Conflicts of interestThe authors declare no conflicts of interest.
The authors thank FAPEMIG, UFMG and FUNED for technical and financial supports.