Meningococcal carriage is a prerequisite for invasive infection. This cross-sectional study assessed the pharyngeal carriage prevalence in healthy subjects aged 1–24 years in Embu das Artes city, São Paulo, Brazil. Pharyngeal swabs were examined for the presence of Neisseria meningitidis. The isolates were tested for different serogroups using agglutination and polymerase chain reaction. A logistic regression model assessed any independent association between Neisseria meningitidis carriage and various risk factors. A total of 87/967 subjects (9%, 95% Confidence Interval (CI): 7.3–11.0) tested positive for N. meningitidis: 6.2% (95% CI: 3.8–9.4) in 1–4 years, 8.5% (95% CI: 5.1–13.0) in 5–9 years, 12.5% (95% CI: 7.8–18.6) in 10–14 years, 12.6% (95% CI: 7.4–19.7) in 15–19 years and 9% (95% CI: 4.9–14.9) in 20–24 years age groups. Highest carriage prevalence was observed in adolescents 10–19 years old. Serogroup C was predominant (18.4%) followed by serogroup B (12.6%). The 15–19 years age group showed a significant association between number of household members and carriers of N. meningitidis. This cross-sectional study is the first in Brazil to evaluate meningococcal carriage prevalence and associated factors in a wide age range.
Meningococcal disease is a serious, rapidly developing, and potentially fatal disease affecting people worldwide with varying incidence depending on the geographical area.1 Around 1.2 million cases of meningococcal infection occur annually with 135,000 related deaths.2Neisseria meningitidis is one of the major causes of bacterial meningitis globally.3 Pharyngeal carriage of N. meningitidis has been considered to be a prerequisite for the development of invasive meningococcal disease (IMD) and known to be essential for transmission.4 The exact mechanism by which a pharyngeal colonization changes into an invasive disease status is not entirely clear. The possible mechanisms that have been suggested are bacterial virulence factors, host susceptibility, including age, prior viral infection, and smoking may ultimately lead to invasive meningococcal disease.5,6N. meningitidis serogroups A, B, C, W, Y, and X account for the majority of IMD cases.2 Globally, meningococcal carriage rates between 5% and 24% have been reported for children and adolescents.7
Meningococcal disease is endemic in Brazil and 1.5–2.0 cases per 100,000 inhabitants were reported during 2000–2009.8 Outbreaks of IMD caused by various serogroups have been reported.9,10 Since 2002, the proportion of cases attributed to meningococcal serogroup C has increased significantly and it is now the most frequent serogroup.1,11,12 After this serogroup C epidemic, Brazil included the meningococcal C conjugate vaccine into the National Immunization Program in 2010.1,12 In addition, studies describing meningococci carriage in Brazil are limited, with few specific studies in adolescents and young adults.8,12–14 No recent data are available on carriage across different age groups from infancy to adulthood and this remains a key knowledge gap. Further data are needed to help understand both the serogroup distribution and disease transmission as they may be useful to help identifying the age groups with higher carriage prevalence rates that could be targeted for vaccination against meningococcal disease and to assess the impact of current vaccination strategies. This information is crucial to support the use of monovalent meningococcal C conjugate vaccine and to gauge if there is a need for another meningococcal vaccine in the country. In this perspective, this cross-sectional study was conducted to assess the prevalence of N. meningitidis carriage and associated factors in among children and adolescents aged 1–24 years.
Materials and methodsStudy design and subjectsThis was a cross-sectional study (GSK study identifier: 113609) conducted in one co-ordinating center, Universidade Federal de São Paulo, in partnership with the Municipal Secretariats of Health and Education in the city of Embu das Artes, São Paulo State, Brazil. Embu das Artes had a population of 240,230 in 2010, with 75% living in the suburbs, and 25% in the city center.15 The city municipality has 14 Basic Healthcare Units (UBS). Subjects were recruited from six UBS (four in suburbs and two in city center). Also, students from primary (6–14 years) and secondary grades (15–18 years) from three schools were invited; two schools in the suburbs and 1 in the city center. Each subject received a single visit.
At the time of enrolment, subjects aged 1–24 years were stratified by age into five groups, i.e. 1–4 years, 5–9 years, 10–14 years, 15–19 years and 20–24 years. This study is part of a larger study assessing seroprevalence of hepatitis A virus (HAV), varicela-zoster virus (VZV), and meningococcal carriage. For the computation of sample size, HAV seroprevalence per age group was considered. After adjusting for 10% non-evaluable subjects, it was planned to enroll 1000 subjects (333 subjects in 1–4 years, 222 in 5–9 years, 167 in 10–14 years, 139 each in 15–19 years and 20–24 years age group) in order to have 900 evaluable subjects (300, 200, 150, 125, and 125 subjects in 1–4, 5–9, 10–14, 15–19 and 20–24 years age group, respectively). Once the target number was reached in a particular age group, enrolment was to be terminated for that age group. The study was conducted from October 2011 to May 2012.
All subjects aged 1–24 years, living in Embu das Artes, São Paulo were eligible to be enrolled in the study. The study was approved by the Ethics Committee of Universidade Federal de São Paulo/Hospital São Paulo, São Paulo, Brazil and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Written informed consent was obtained from the subject/parents/legally acceptable representatives of the subject prior to the performance of any study-specific procedure. A written informed assent was obtained from subjects aged >10 years and below the legal age of consent according to local regulations.
AssessmentsRecording of demographic and socioeconomic variablesThe subject or parents/legally acceptable representatives of the subjects enrolled in the study were provided a questionnaire to inquiring about age, gender, area of residence, socioeconomic factors, and previous history of medical and meningococcal vaccination. The socioeconomic factors included family income (total or per capita), number of household members, attendance to an educational establishment (public or private), and type of health system used by subjects (public or private).
Laboratory assaysA pharyngeal alginate swab was collected from the posterior pharyngeal wall of each subject and placed in a tube with 1-mL Skim Milk Tryptone Glucose Glycerine (STGG) transport medium and sent to the central laboratory of Universidade Federal de São Paulo at room temperature (20–26°C) within five hours for subsequent analysis. An aliquot of 100μL was plated onto selective Thayer-Martin medium modified with VCNT (vancomycin, colistin, nystatin and trimethoprim). All swab specimens were tested using latex agglutination for species identification and serogroups A, B, C, W, and Y. In parallel, real-time polymerase-chain reactions (qPCR) for meningococcal identification and genogroups A, B, C, W, X and Y were performed at Instituto Adolfo Lutz for all swab samples.
DNA extraction and quantitative PCRAn aliquot of 200μL from pharyngeal swab samples were extracted and purified using a MagNA Pure LC Robot Instrument with LC MagNA Pure Nucleic Acid Isolation kit I from Roche Applied Science, according to the manufacturer's instructions. In this study, qPCR amplification was performed by TaqMan probe systems for the detection of N. meningitidis ctrA, sodC, and capsular biosynthesis genes as targets for A, B, C, W, X, and Y serogroups of N. meningitidis in all positive samples. All qPCR reactions were performed using the 7500 ABI PCR platform (Applied Biosystems, USA). Samples were deemed positive when showing an amplification curve and multi-component characteristics with a cycle threshold cut-off value ≤38.16,17
Statistical analysisAll analyses included subjects who met the eligibility criteria and complied with all the procedures. The study assessed if N. meningitidis carriage was associated with any socioepidemiological and clinical characteristics such as socioeconomic status, vaccination status, type of health system and educational facility used, antibiotic use, and history of meningitis using a logistic regression model.
The variables gender, per capita income (low/medium/high), surrogate variable for socioeconomic status (SES), and risk factors (whether the child attended day care centers, attendance in school, education level, number of household members, vaccination status, health insurance, disease history, previous antibiotic usage, etc.) were included as independent variables for each age-group. This multiple logistic regression was used to assess the independent association between Neisseria meningitidis carriage and selected risk factors. Using the backward selection strategy (with p-value of 0.20 to stay in the model – SLSTAY=0.20) and the method of Maximum Likelihood to estimate the parameters, the final model identified the risk factors significantly associated with carriage.
ResultsOverall pharyngeal carriage prevalence of N. meningitidisA total of 968 subjects provided informed consent. Of those, 967 pharyngeal swabs were available, one sample missing in the 15–19 year group. Sociodemographic distributions of subjects are shown in Table 1. The prevalence of N. meningitidis pharyngeal carriage by age group is presented in Table 2.
Sociodemographic characteristics of study subjects.
| N (total=967) | (%) | ||
|---|---|---|---|
| Age group | 1–4 years | 323 | 33.4 |
| 5–9 years | 213 | 22.1 | |
| 10–14 years | 160 | 16.5 | |
| 15–19 years | 127 | 13.1 | |
| 20–24 years | 144 | 14.9 | |
| Gender | Female | 497 | 51.4 |
| Male | 470 | 48.6 | |
| SES | Low | 907 | 93.8 |
| Medium | 52 | 5.4 | |
| Unknown | 8 | 0.8 | |
| Health System used | Public | 138 | 97.0 |
| Private | 24 | 2.5 | |
| N.A. | 5 | 0.5 | |
| Educational facility used | Public | 176 | 18.2 |
| Private | 20 | 2.0 | |
| N.A. | 771 | 79.8 | |
Prevalence of positive N. meningitidis carriage by age group.
| 1–4 years N=323 | 5–9 years N=213 | 10–14 years N=160 | 15–19 years N=127 | 20–24 years N=144 | Total N=967 | |
|---|---|---|---|---|---|---|
| n (%) 95% CI | n (%) 95% CI | n (%) 95% CI | n (%) 95% CI | n (%) 95% CI | n (%) 95% CI | |
| Positive for N. meningitidis | 20 (6.2) 3.8–9.4 | 18 (8.5) 5.1–13.0 | 20 (12.5) 7.8–18.6 | 16 (12.6) 7.4–19.7 | 13 (9) 4.9–14.9 | 87 (9) 7.3–11.0 |
N, total number of subjects in a given age category; n, number of subjects in a given laboratory result category; 95% CI, 95% confidence interval.
Out of the 967 subjects, there were 87 carriers of N. meningitidis [9.0%; 95% confidence interval (CI): 7.3–11.0%]. Higher percentages of positive subjects per age group were observed in the age categories of 15–19 (12.6%, 95% CI: 7.4–19.7) and 10–14 (12.5%, 95% CI: 7.8–18.6) years, respectively (Table 3). The majority belonged to mixed races (n=84), one subject was of African/African-American heritage, while two were of Caucasian/European heritage.
Pharyngeal carriage of N. meningitidis by socioepidemiological and clinical characteristics.
| Characteristics | Categories | 1–4 years (n=20/N=323) | 5–9 years (n=18/N=213) | 10–14 years (n=20/N=160) | 15–19 years (n=16/N=127) | 20–24 years (n=13/N=143) | Total (n=87/N=967) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n/N (%) | 95% CI | n/N (%) | 95% CI | n/N (%) | 95% CI | n/N (%) | 95% CI | n/N (%) | 95% CI | n/N (%) | 95% CI | ||
| Gender | Female | 7/139 (5.0) | (2.0–10.4) | 9/106 (8.5) | (3.9–16.1) | 12/70 (17.1) | (8.9–30.0) | 10/77 (13.0) | (6.2–23.9) | 9/105 (8.6) | (3.9–16.3) | 47/497 (9.5) | (7.0–12.6) |
| Male | 13/184 (7.1) | (3.8–12.1) | 9/107 (8.4) | (3.9–16.0) | 8/90 (8.9) | (3.8–17.5) | 6/50 (12.0) | (4.4–26.1) | 4/39 (10.3) | (2.8–26.3) | 40/470 (8.5) | (6.1–11.6) | |
| SES | Low | 20/312 (6.4) | (3.9–9.9) | 18/205 (8.8) | (5.2–13.9) | 20/155 (12.9) | (7.9–19.9) | 15/117 (12.8) | (7.2–21.2) | 10/118 (8.5) | (4.1–15.6) | 83/907 (9.2) | (7.3–11.3) |
| Medium | 0/11 (0.0) | (0.0–33.5) | 0/7 (0.0) | (0.0–52.7) | 0/5 (0.0) | (0.0–73.8) | 0/5 (0.0) | (0.0–73.8) | 3/24 (12.5) | (2.6–36.5) | 3/52 (5.8) | (1.2–16.9) | |
| Unknown | 0/0 (–) | (0.0–100.0) | 0/1 (0.0) | (0.0–368.9) | 0/0 (–) | (0.0–100.0) | 1/5 (20.0) | (0.5–111.4) | 0/2 (0.0) | (0.0–184.4) | 1/8 (12.5) | (0.3–69.7) | |
| Health system useda | Public | 19/314 (6.1) | (3.6–9.5) | 18/208 (8.7) | (5.1–13.7) | 20/156 (12.8) | (7.8–19.8) | 16/125 (12.8) | (7.3–20.8) | 13/135 (9.6) | (5.1–16.5) | 86/938 (9.2) | (7.3–11.3) |
| Private | 0/7 (0.0) | (0.0–52.7) | 0/3 (0.0) | (0.0–123.0) | 0/4 (0.0) | (0.0–92.2) | 0/1 (0.0) | (0.0–368.9) | 0/9 (0.0) | (0.0–41.0) | 0/24 (0.0) | (0.0–15.4) | |
| N.A. | 1/2 (50.0) | (1.3–278.6) | 0/2 (0.0) | (0.0–184.4) | 0/0 (–) | (0.0–100.0) | 0/1 (0.0) | (0.0–368.9) | 0/0 (0.0) | (0.0–100.0) | 1/5 (20.0) | (0.5–111.4) | |
| Educational facility usedb | Public | 7/142 (4.9) | (2.0–10.2) | 3/34 (8.8) | (1.8–25.8) | 0/0 (–) | (0.0–100.0) | 0/0 (–) | (0.0–100.0) | 0/0 (–) | (0.0–100.0) | 10/176 (5.7) | (2.7–10.5) |
| Private | 1/17 (5.9) | (0.2–32.8) | 0/3 (0.0) | (0.0–123.0) | 0/0 (–) | (0.0–100.0) | 0/0 (–) | (0.0–100.0) | 0/0 (–) | (0.0–100.0) | 1/20 (5.0) | (0.1–27.9) | |
| N.A. | 12/164 (7.3) | (3.8–12.8) | 15/176 (8.5) | (4.8–14.1) | 20/160 (12.5) | (7.6–19.3) | 16/127 (12.6) | (7.2–20.5) | 13/144 (9.0) | (4.8–15.4) | 76/771 (9.9) | (7.8–12.3) | |
| Vaccination status | Yes | 8/167 (4.8) | (2.1–9.4) | 0/1 (0.0) | (0.0–368.9) | 0/3 (0.0) | (0.0–123.0) | 2/3 (66.7) | (8.1–240.8) | 0/0 (–) | (0.0–100.0) | 10/174 (5.8) | (2.8–10.6) |
| No | 12/142 (8.5) | (4.4–14.8) | 18/212 (8.5) | (5.0–13.4) | 20/157 (12.7) | (7.8–19.7) | 14/123 (11.4) | (6.2–19.1) | 13/144 (9.0) | (4.8–15.4) | 77/778 (9.9) | (7.8–12.4) | |
| Unknown | 0/14 (0.0) | (0.0–26.4) | 0/0 (–) | (0.0–100.0) | 0/0 (–) | (0.0–100.0) | 0/1 (0.0) | (0.0–368.9) | 0/0 (–) | (0.0–100.0) | 0/15 (0.0) | (0.0–24.6) | |
| History of Meningitis | Yes | 0/1 (0.0) | (0.0–368.9) | 0/1 (0.0) | (0.0–368.9) | 0/2 (0.0) | (0.0–184.4) | 0/1 (0.0) | (0.0–368.9) | 0/2 (0.0) | (0.0–184.4) | 0/7 (0.0) | (0.0–52.7) |
| No | 20/322 (6.2) | (3.8–9.6) | 18/212 (8.5) | (5.0–13.4) | 20/158 (12.7) | (7.7–19.6) | 16/123 (13.0) | (7.4–21.1) | 13/140 (9.3) | (4.9–15.9) | 87/955 (9.1) | (7.3–11.2) | |
| Unknown | 0/0 (–) | (0.0–100.0) | 0/0 (–) | (0.0–100.0) | 0/0 (–) | (0.0–100.0) | 0/3 (0.0) | (0.0–123.0) | 0/2 (0.0) | (0.0–184.4) | 0/5 (0.0) | (0.0–73.8) | |
| History of meningitis and contacts with meningitis affected patients | Yes | 1/4 (25.0) | (0.6–139.3) | 1/6 (16.7) | (0.4–92.9) | 1/3 (33.3) | (0.8–185.7) | 0/4 (0.0) | (0.0–92.2) | 1/7 (14.3) | (0.4–79.6) | 4/24 (16.7) | (4.5–42.7) |
| No | 19/318 (6.0) | (3.6–9.3) | 17/205 (8.3) | (4.8–13.3) | 19/151 (12.6) | (7.6–19.6) | 15/118 (12.7) | (7.1–21.0) | 12/130 (9.2) | (4.8–16.1) | 82/922 (8.9) | (7.1–11.0) | |
| Unknown | 0/1 (0.0) | (0.0–368.9) | 0/2 (0.0) | (0.0–184.4) | 0/6 (0.0) | (0.0–61.5) | 1/5 (20.0) | (0.5–111.4) | 0/7 (0.0) | (0.0–52.7) | 1/21 (4.8) | (0.1–26.5) | |
| Use of antibiotics | Yes | 6/106 (5.7) | (2.1–12.3) | 2/35 (5.7) | (0.7–20.6) | 0/16 (0.0) | (0.0–23.1) | 2/17 (11.8) | (1.4–42.5) | 0/19 (0.0) | (0.0–19.4) | 10/193 (5.2) | (2.5–9.5) |
| No | 13/214 (6.1) | (3.2–10.4) | 16/178 (9.0) | (5.1–14.6) | 20/144 (13.9) | (8.5–21.5) | 14/109 (12.8) | (7.0–21.6) | 13/123 (10.6) | (5.6–18.1) | 76/768 (9.9) | (7.8–12.4) | |
| Unknown | 1/3 (33.3) | (0.8–185.7) | 0/0 (–) | (0.0–100.0) | 0/0 (–) | (0.0–100.0) | 0/1 (0.0) | (0.0–368.9) | 0/2 (0.0) | (0.0–184.4) | 1/6 (16.7) | (0.4–92.9) | |
N, number of subjects for whom the test was performed; n, number of N. meningitidis positive subjects in that category; 95% CI, 95% confidence interval; SES, socio economic status; N.A., not applicable.
Low, per capita value ≤559 BRL (Brazilian Real); medium, per capita value between 560 and 1120 BRL; high, per capita value ≥1121 BRL.
Of the 87 carriers, ctrA and sodC target genes were detected in 58 (6%) whereas in 29 (3%) only sodC was detected. Of those58 positive samples 32 were characterized as genogroups C (16; 50%), B (11; 34%), Y (4; 13%), X (1; 3%), and 26 were negative in the genogrouping assay. In total, 5.8% of samples had N. meningitidis with invasive properties and 2.9% had no invasive power.
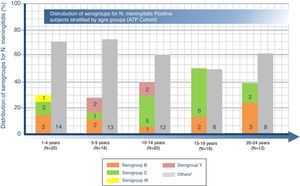
Distribution of serogroups for N. meningitidis-positive subjects by age groupOverall, among the 87 carriers, N. meningitidis serogroups C and B were the most common with positivity rates of 18.4% (95% CI: 10.9–28.1) and 12.6% (95% CI: 6.5–21.5), respectively. The highest percentage of serogroup B was found in the age group 20–24 years (23.1%, n=3), serogroup C to in the age group 15–19 years (37.5%, n=6), and serogroup Y in group 5–9 years (11.1%, n=2) as shown in Fig. 1. Furthermore, serogroups W, X, and E were identified in one subject each (Fig. 1). Another 53 (60.9%) subjects were non-groupable, i.e. negative in the genogrouping assay for N. meningitidis serogroups A, B, C, W, Y, and X, but positive for N. meningitidis. No cases with serogroup A were identified.
Number (n) of N. meningitidis positive subjects in each serogroup stratified by age range.
N=total number of N. meningitidis positive subjects in a given age category.
“Others” includes non-groupable and serogroups E and X. (Serogroups E and X had 1 subject each in 5–9 years and 20–24 year age groups, respectively.).
Results are summarized in Table 3.
Positivity rate by SESThe majority of the N. meningitidis-positive subjects fell under low SES group (n/N=83/907) as shown in Table 3.
Positivity rate by health system (type of health insurance) and educational facility usedOverall, 9.2% (95% CI: 7.3–11.3) of the 938 subjects using the public health system were positive and subjects in the age groups 10–14 and 15–19 years recorded the highest pharyngeal carriage prevalence of 12.8% each (95% CIs: 7.8–19.8 and 7.3–20.8, respectively) (Table 2). A total of 196 subjects aged ≤9 years attended an educational establishment; 176 and 20 subjects used a public and private educational facility, respectively. Of these, 5.7% (95% CI: 2.7–10.5) and 5% (95% CI: 0.1–27.9) were found positive, respectively. As per age group distribution, 4.9% (95% CI: 2.0–10.2) and 8.8% (95% CI: 1.8–25.8) subjects using a public educational establishment tested positive in the age groups 1–4 years and 5–9 years, respectively. The only subject who used a private educational establishment and tested positive for N. meningitidis was in the 1–4 year group (Table 3).
By vaccination statusAmong 967 subjects (174 vaccinated, 778 not vaccinated, and 15 subjects with unknown vaccination status), 10 (5.75%; 95% CI: 2.76–10.57) were vaccinated against meningococcal disease (Table 2). Out of 127 subjects (three vaccinated, 123 non vaccinated and one subject with unknown vaccination status) carrying N. meningitidis, the highest percentage of subjects vaccinated against meningococcal disease were in the age group 15–19 years (n=2; 66.7%; 95% CI: 8.1–240.8) (Table 2). Of the 174 vaccinated subjects, 10 (5.8%; 95% CI: 2.8–10.6) were positive and a large majority (n=167; 95.9%) were in the age group 1–4 years with a N. meningitidis pharyngeal carriage prevalence of 4.8% (95% CI: 2.1–9.4) vs. 8.5% in unvaccinated subjects.
By history of meningitis and contact with a meningitis affected patientAmong the 967 enrolled subjects, seven subjects had a previous history of meningitis and none of them tested positive for N. meningitidis carriage (Table 2). Otherwise, out of 24 subjects who had contacts with meningitis-affected patients, four (16.7%; 95% CI: 4.5–42.7) showed evidence of bacteria carriage. Age distribution of subjects is shown in Table 2.
By use of antibioticsTen of the 193 subjects (5.2%) who took antibiotics (rifampin, ciprofloxacin, ceftriaxone, cefotaxime, moxifloxacin, levofloxacin, or azitromycin) in the three months prior to inclusion were positive for N. meningitidis. The highest pharyngeal carriage prevalence of N. meningitidis was observed in the age group 15–19 years (11.8%). However, the statistical significance of this finding is uncertain as a result of overlapping confidence intervals (95% CI: 1.4–42.5) (Table 3).
Regression analysisA logistic regression model on seroprevalence considering gender, per capita income, and surrogate variable for socioeconomic status, antibiotic use, and risk factors as independent variables was constructed by age group in accordance with the study objectives (Table 4). Among the various risk factors studied for N. meningitidis carriage, a significant association was found for the number of positive household members in the age group 15–19 years (p≤0.02). The risk of pharyngeal carriage increased with the number of household members considered as a continuous variable in the logistic regression model (Odds Ratio: 1.35; 95% CI: 1.1–1.7).
Risk factors by age group independently associated with N. meningitidis carriage in multiple logistic regression.
| Age group | Model section | Characteristics | p-value | Odds ratio (OR)a | 95% CI of OR | |
|---|---|---|---|---|---|---|
| LL | UL | |||||
| 1–4 years | Saturated model | Number of household members | 0.2993 | 1.135 | 0.894 | 1.441 |
| Gender (female vs male) | 0.6136 | 0.777 | 0.292 | 2.070 | ||
| Day care centers (private vs public) | 0.7688 | 0.659 | 0.041 | 10.656 | ||
| Vaccination status (yes vs no) | 0.1340 | 0.480 | 0.184 | 1.254 | ||
| Previous antibiotic usage (yes vs no) | 0.8837 | 1.079 | 0.389 | 2.991 | ||
| Final model | Previous antibiotic usage (yes vs no) | 0.8751 | 0.923 | 0.340 | 2.504 | |
| 5–9 years | Saturated model | Number of household members | 0.3581 | 1.129 | 0.872 | 1.462 |
| Gender (female vs male) | 0.9909 | 1.006 | 0.382 | 2.649 | ||
| Previous antibiotic usage (yes vs no) | 0.4675 | 0.564 | 0.120 | 2.642 | ||
| Final model | Nil | |||||
| 10–14 years | Saturated model | Number of household members | 0.1570 | 1.179 | 0.939 | 1.480 |
| Gender (female vs male) | 0.1193 | 2.155 | 0.820 | 5.660 | ||
| Scholarity level (secondary vs basic) | 0.7926 | 1.149 | 0.408 | 3.239 | ||
| Scholarity level (high school vs basic) | 0.9833 | 0.000 | <0.001 | . | ||
| Final model | Number of household members | 0.1590 | 1.179 | 0.937 | 1.484 | |
| Gender (female vs male) | 0.1245 | 2.128 | 0.812 | 5.574 | ||
| 15–19 years | Saturated model | Number of household members | 0.0186 | 1.349 | 1.051 | 1.731 |
| Gender (female vs male) | 0.9336 | 1.051 | 0.324 | 3.414 | ||
| Employment (informal vs formal) | 0.5018 | 2.173 | 0.226 | 20.926 | ||
| Vaccination status (yes vs no) | 0.0702 | 15.240 | 0.799 | 290.845 | ||
| Previous antibiotic usage (yes vs no) | 0.5268 | 0.564 | 0.095 | 3.327 | ||
| Final model | Number of household members | 0.0316 | 1.301 | 1.023 | 1.653 | |
| Vaccination status (yes vs no) | 0.0349 | 14.998 | 1.212 | 185.593 | ||
| 20–24 years | Saturated model | Number of household members | 0.1871 | 0.751 | 0.491 | 1.149 |
| Gender (female vs male) | 0.7601 | 0.818 | 0.225 | 2.972 | ||
| Monthly family per capita income (medium vs low) | 0.8753 | 1.137 | 0.229 | 5.632 | ||
| Employment (informal vs formal) | 0.3723 | 2.052 | 0.423 | 9.955 | ||
| Final model | Number of household members | 0.1500 | 0.744 | 0.497 | 1.113 | |
95% CI, 95% confidence interval of odds ratio; LL, lower limit; UL, upper limit.
Note 1: Final model is based on the backward elimination strategy using logistic regression with SLSTAY=0.20; Note 2: some of the independent variables were not included in the model due to lack of observations.
Four epidemic outbreaks have been reported in São Paulo in the last century. The first two outbreaks, occurring in 1920 and 1944, were caused by meningococcal serogroup A.18,19 The third (serogroup C – 1971) and fourth (serogroup A – 1974) outbreaks were characterized by two overlapping epidemic waves. Until then, the prevalence of serogroup C had not been associated with major outbreaks. This epidemic provided the impetus for first major mass testing of polysaccharide A and C vaccines resulting in control of the epidemic by 1975.9,18,19 In the late 1980s, epidemics due to serogroup B occurred in several locations across the country, demonstrating the highly variable and unpredictable epidemiology of this disease.
Since 2002, a significant increase in the proportion of cases attributed to serogroup C has been observed in São Paulo20 and other regions21 across Brazil making it in 2010 the most frequent serogroup causing meningococcal disease in the country.8,9 A meningococcal C conjugate vaccine (MCC) was introduced to the Brazilian Immunization Program in December 2010 for children under two years of age.
Despite the importance of N. meningitidis carriage for transmitting the disease, limited data describing N. meningitidis carriage in Brazil are available.8,12,13 Looking for covering this gap, this study assessed the N. meningitidis carriage in a wider age range, from childhood to young adult from a selected city of São Paulo state.
In this study, the overall carriage prevalence observed was similar to those observed in other studies.8,11,13 Although the carriage rate in children was not negligible, the highest carriage prevalence was observed in adolescents 10–19 years old.
Two years after the introduction of meningococcal C conjugate vaccination program in Brazil (2011–2012), high vaccine coverage (90–95%) was achieved, and a 50% reduction in the incidence of disease in the vaccinated cohort was demonstrated. However, no impact was observed in other age groups.12 Clearly, vaccinating only young children, who are not the main reservoir of N. meningitidis, will not result in herd protection.12,22,23 Our study results show that the highest carriage prevalence has been observed in older adolescents and young adults, similar to other studies.7,13,24 This age group should be included in a catch-up vaccination program, to achieve herd protection and successful meningococcal C disease control.
This study started 10 months after the introduction of the MCC vaccine in the National Immunization Program for children under two and some of the participants have received MCC vaccine. We observed a higher prevalence of pharyngeal carriage among unvaccinated (8.5%) than among vaccinated subjects (4.8%). MCC vaccination prevents acquisition of carriage, which is not observed with polysaccharide vaccines.8
In our study sample, serogroup C was the dominant serogroup followed by serogroup B. These results are aligned with another recent study on 11–19 year-old adolescents in Campinas, Brazil where serogroup C was also the most prevalent serogroup.25 However, differences in prevalence of serogroups across geographical regions have been observed. While recent studies on meningococcal carriage in Europe24 and particularly in United Kindom26 have identified serogroup B as the most prevalent serogroup, in another study conducted in Africa it was serogroup W.27,28 These data suggest a regional variation in serogroup meningococcal carriage.
In this study the pharyngeal carriage prevalence of N. meningitidis was also evaluated on the basis of different risk factors. Higher pharyngeal carriage prevalence was observed in previously unvaccinated subjects, who used the public health system, and attended public educational institutions. Higher pharyngeal carriage prevalence was also seen in subjects with no previous history of meningitis, those with a previous history of contact with a meningitis-affected patient, and those who had not used antibiotics previously. However, no significant association (p≤0.05) was observed between carriage prevalence and risk factors in the multivariate analysis. Nonetheless, an increase in the number of household members was found to be independently associated with an increased risk of meningococcal carriage in the logistic regression analyses.
The strength of this study is that it was population-based where subjects across a wide age-range were recruited from different facilities like health care centers, day care centers and schools thereby covering a wider population and giving better surveillance. Further, qPCR, a sensitive technique, was used in this study for detection of different serogroups. A limitation of this study is that the selected population constitutes a convenience sample and the results cannot be extrapolated to all communities in Brazil and its external validity may be subject to criticism. However, the results found by age-group are consistent with those reported in the literature.29–31 A low sample size in some age groups could have also been a limitation of this study. The fact that we did not assessed the association between smoking habits of older subjects (or household members) and risk of N. meningitidis carriage constitutes another limitation. As mentioned earlier, 60.9% of meningococci carriers (n=53) identified in the current study remained non-groupable, i.e. not capsulated. However, this should not be seen as a limitation of the study. In fact, most carrier isolates have been shown to lack capsule production. Approximately 50% of the strains isolated from carriers lack capsule and are therefore serologically not groupable.32,33 In one of the studies on meningococcal carriage prevalence in Chile, 65% of isolated strains were non-groupable.34 Similar high percentages of non-groupable strains have been reported in other studies.8,35
This was the first study in Brazil evaluating the meningococcal carrier state in a wide age range. This study revealed that both the age groups 10–14 and 15–19 years had similarly high pharyngeal carriage prevalence of N. meningitidis in Embu das Artes. Vaccination programs should consider the inclusion of catch-up cohorts to achieve herd protection with direct and indirect effects of vaccination. Serogroup C was the most predominant, followed by serogroup B, and to a lesser extent serogroups Y and W. Due to the impact of meningococcal C vaccination and highly variable and unpredictable epidemiology of meningococcal disease, a constant surveillance is required. This study will further add to our understanding of meningococcal disease epidemiology in Brazil and may assist in developing future vaccination strategies.
AuthorshipAll authors participated in the design or implementation or analysis, and interpretation of the study and in the development of this manuscript. All authors had full access to the data and gave final approval before submission. The corresponding author was responsible for submission of the publication.
Conflicts of interestL.Y. Weckx received Institutional grants from the GSK group of companies during the conduct of the study. E. Ortega-Barria, R Colindres, E.N.C. Barros and R. Devadiga are employees of the GSK group of companies. E. de Barros, E. Ortega-Barria and R Colindres own stock options in the GSK group of companies. S.H. Tuboi was a former employee of the GSK group of companies at the time of the study and owns restricted shares in the GSK group of companies. M.G. Gonçalves, R.F. Puccini and A.M.O. Machado have nothing to disclose.
The authors thank all study participants and their families, all clinical study site personnel who contributed in conducting this trial, and the following persons: David Fermin Arguello, Marcia Aparecida Pozo Pereira, Ana Paula Silva de Lemos, Maria Cecilia O Gorla and Maria Cristina C Brandileone. The authors would also like to thank the Business & Decision Life Sciences platform for editorial assistance and manuscript coordination on behalf of GSK Vaccines. Pierre-Paul Prévot (Business & Decision Life Sciences) and Stéphanie Delval (Consultant for XPE Pharma & Science) coordinated the manuscript development and provided editorial support. Maurice Rouillard (Business & Decision Life Sciences), Ramandeep Singh and Sasi Taneja (GSK) provided medical writing assistance.
GlaxoSmithKline Biologicals SA was the funding source and was involved in all stages of the study conduct and analysis. GlaxoSmithKline Biologicals SA also funded all costs associated with the development and the publishing of the present manuscript.