An outbreak of acute hepatitis of unknown etiology in children was recently reported worldwide. We aimed to describe the burden of hospitalizations due to hepatitis of unknown etiology in children/adolescents in Brazilian public hospitals.
MethodsWe retrieved a database of all hospitalizations in the Brazilian Unified Health System (SUS) from January/2019 to February/2022 using the “microdatasus” R package. Hepatitis of unknown etiology was defined by the following International Classification of Diseases [ICD-10] codes: B19, B19.0, B19.9, K72.0, K72.9, K75, K75.9, R94.5, or R93.2. The incidence rates (95% confidence interval, IC) per 1,000 all-cause hospitalizations in different age strata [< 6 years; 6–11 years and 12–17 years] were estimated.
ResultsA total of 94,198 hospitalizations due to hepatic or infectious diseases with potential liver injury were analyzed. Of them, 1,535 children/adolescents [48.2% male sex, 41.6% aged < 6 years] were hospitalized with hepatitis with unknown etiology. The top ICD-10 codes were B19.9 [unspecified viral hepatitis without hepatic coma; 39.9% (n = 612)], K72.9 [hepatic failure, unspecified; 29.8% (n = 457)], and K72.0 [hepatic failure, not elsewhere classified; 14.5% (n = 223)]. A total of 8.5% (n = 131) of individuals required liver transplantation and 7.0% (n = 107) died during the hospital-stay. In 2021, the incidence rates (95% CI) of hospitalizations for hepatitis with unknown etiology were 7.80 (7.63–7.98), 17.96 (17.46–18.48) and 13.28 (12.95–13.62) per 1,000 all-cause hospitalizations in subjects aged < 6 years, 6–11 years and 12–17 years-old, respectively. Similarly, the incidence rates of hospitalization due to hepatitis with unknown etiology per 1,000 all-cause hospitalizations (CI95%) in January-February/2022 were 7.52 (7.11–7.94), 16.82 (15.68–18.03), and 13.96 (13.10–14.85) for children/adolescents with age < 6 years, 6–11 years, and 12–17 years, respectively.
ConclusionsA non-negligible number of hospitalizations due to hepatitis with unknown etiology in children/adolescents was observed in the last years in Brazil. Up to 15% of those cases needed liver transplantation or died.
Recently, a multicountry outbreak of acute hepatitis of unknown etiology in children aged 16 years or below has been reported by the World Health Organization (WHO) and health agencies.1 A person aged 16 years old or younger having an acute hepatitis (non A-E hepatitis viruses) with aspartate transaminase (AST) or alanine transaminase (ALT) over 500 IU/L, since October 1st 2021 could be considered a probable case of acute hepatitis of unknown etiology. Mostly, those children had gastrointestinal symptoms, such as abdominal pain, diarrhea, and/or vomiting, followed by a flare of transaminases and jaundice without any significant past medical history.2 As of 12 July 2022, 35 countries in five WHO Regions had reported 1010 probable cases of severe acute hepatitis of unknown etiology in children. Most cases were reported in the WHO European Region (n = 484) and Region of Americas (n = 435), including 334 cases (33% of global cases) in the United States of America.3 As of June 30th 2022, a total of 473 cases were reported in 21 countries by WHO Regional Office for Europe, mainly in the United Kingdom (n = 268).4 Epidemiological data remain scarce in Latin America. As of June 29th 2022, a total of 163 suspected cases of acute hepatitis of unknown etiology were reported by local health authorities to the Brazilian Ministry of Health (BMoH). Of which, two probable cases, seven suspected, one child lost to follow-up, 67 cases considered as unlikely acute hepatitis of unknown etiology, and 86 cases remained under investigation.5
International health authorities continue to investigate suspected cases to determine the disease etiology and to better inform prevention and control measures. However, it is still unclear if there has been an increase in hepatitis cases, or an increase in awareness of hepatitis cases in children that occur at the expected rate but go undetected. Therefore, we aimed to describe the number and incidence rates of children or adolescents’ hospitalization due to hepatitis of unknown etiology, liver diseases with specified causes and viral infections, including adenovirus and SARS-CoV-2, in Brazilian public hospitals from 2019 to 2022.
Material and methodsWe retrieved a database that included all hospitalization information in the Brazilian Unified Health System (SUS) hospitals from the Hospital Information System (SIH-SUS) between January 2019 and February 2022 using the “microdatasus” R package6 for the software (R version: 4.2).7 The SIH-SUS is a publicly available national database from the BMoH where anonymous data concerning all hospitalizations that occurs in the Brazilian Public Health System has been systematically registered in the last decade. The following data of hospitalizations are available in this database: socio-demographic characteristics, geographical location, length of hospitalization, causes of hospitalization (using the International Classification of Diseases, Tenth Revision [ICD-10] codes), procedures (organ and/or tissue transplantation) and outcomes (death during hospital stay) (https://bvsms.saude.gov.br/bvs/publicacoes/07_0066_M.pdf).
A filtering function was used keeping only data of hospitalizations in subjects aged lower than 18 years old. Within this subset, the following etiology classification were used for each hospitalization: (1) hepatitis with unknown etiology: hospitalizations for hepatitis or other liver inflammatory disease without any specific etiology or liver transplantation [ICD-10 codes: B19 (unspecified viral hepatitis), B19.0 (unspecified viral hepatitis with hepatic coma), B19.9 (unspecified viral hepatitis without hepatic coma), K72.0 (hepatic failure, not elsewhere classified), K72.9 (hepatic failure, unspecified), K75 (other inflammatory liver diseases), K75.9 (inflammatory liver disease, unspecified) or R93.2 (abnormal findings on diagnostic imaging of liver and biliary tract)]; (2) viral hepatitis A-E (ICD-10 codes: B15, B15.0, B15.9, B16, B16.0, B16.1, B16.2, B16.9, B17.0, B17.1 or B17.2); (3) other infectious hepatitis (ICD-10 codes: A06.4, B17, B17.8, B25.1, B67.0, B67.5, B67.8, B94.2, O98.4 or P35.3); (4) other liver diseases (ICD-10 codes: K72, K74.1, K75.2, K75.3, K75.4, K75.8, K76, K76.0, K76.1, K76.2, K76.3, K76.4, K76.5, K76.8, K76.9, K77, Q26.6, Q44, Q44.6 or Q44.7); (5) adenovirus infection (ICD-10 codes: A08.2, A85.1, A87.1, B30.0, B30.1, B34.0, B97.0 or J12.0); (6) COVID-19 (ICD-10 codes: B34.2, B97.2, U07.1, U07.2, U09.9, U10.9, U12.9 or U92.1); (7) other viral infection (Chikungunya, Cytomegalovirus, Dengue, Epstein-Barr Virus, Enterovirus, Norovirus, Yellow fever; ICD-10 codes: B25.9, B25, B27.1, B25.8, B25.2, B25.0, A90, A91, D82.3, B30.3, A85.0, B97.1, B08.4, B08.5, A88.0, B34.1, A87.0, A08.1, A95, A95.9, A95.0, A95.1 or Z24.3); and (8) complications of liver transplant (ICD-10 codes: T86.4 or Z94.4). Cases primary defined as hepatitis with unknown etiology but having concomitantly ICD-10 codes of any other disease [viral hepatitis A-E; other infectious hepatitis; other liver diseases; adenovirus infection; COVID-19; other viral infection or complications of liver transplant] were reclassified as the specific disease.
The overall number of hospitalizations of < 18 years old individuals with disaggregation by the three age strata (0 to < 6, 6 to < 12, and 12 to < 18 years old) was determined and used as the denominator to estimate the incidence rate of hospitalizations for the different etiology classifications. Descriptive tables were used to present the results of hospitalizations for hepatitis with unknown etiology and other liver or viral diseases that could be considered possible confounders. Time series plots were developed presenting the incidence rate of hospitalizations for hepatitis with unknown etiology by months between 2019 and February 2022. The incidence rate has the number of patients with hospitalization with unknown etiology as numerator divided by the overall number of hospitalizations within age strata for each month. 95% confidence intervals (CI) were constructed for the hospitalization incidence rates according to asymptotic standard errors calculated from a Gamma distribution.8
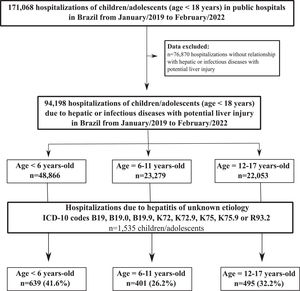
ResultsOverall hospitalizationsA total of 171,068 hospitalizations of children/adolescents (age < 18 years) from January/2019 to February/2022 were identified. Of them 94,198 hospitalizations due to hepatic or infectious diseases with potential liver injury were analyzed (Fig. 1). Overall, 53.2% of hospitalized children/adolescents were male, 51.9% were aged less than six years, 45.4% with bi-racial (“Pardo”) skin-color, and 42.4% occurred in the Northeast region. SARS-CoV-2 infection (52.7%, n = 49,293) and Dengue (33.9%, n = 31,743) were the most prevailing causes of hospitalization; up to 2.0% (n = 1844) of children/adolescents were hospitalized due to an Adenovirus infection. The mean length of hospitalization was 5.38 (SD, 6.53) days and 2.0% (n = 1867) of individuals died during hospitalization. Table 1 describes the characteristics of children/adolescents hospitalized from January/2019 to February/2022. Liver diseases were responsible for 7.6% (n = 7182/94,198) of hospitalizations; hepatitis with unknown etiology represented 1.6% (n = 1535) of total hospitalizations, and 21.4% of hospitalizations were due to other liver diseases. Viral hepatitis A-E were responsible for 0.5% (n = 467) of total hospitalizations, mainly driven by hepatitis A infection (n = 283). Additionally, 0.5% (n = 511) were hospitalized due to toxic hepatic disease; 0.6% (n = 552) due to autoimmune hepatitis, and 1.1% (n = 1009) due to complications of liver transplant (Table 2). A total of 73 individuals were primary classified as hepatitis of unknown etiology, but they had a secondary ICD-10 code referring to a specific disease [complications of liver transplant (n = 55), hepatic impairment (n = 5), auto-immune hepatitis (n = 3), reactive hepatitis (n = 3), toxic liver disease (n = 2), cytomegalovirus infection (n = 2), hepatitis A (n = 1), enterovirus (n = 1), and other acute viral hepatitis (n = 1)].
Characteristics of children/adolescents (< 18 years-old year) hospitalized from January/2019 to February/2022 in the Brazilian Public Health System.
Data expressed as a number (%) and b mean (standard-deviation); †p-values obtained by chi-square tests. Disease categories were classified based on International Classification of Diseases (ICD-10) codes as hepatitis of unknown etiology (ICD-10: B19, B19.0, B19.9, K72.0, K72.9, K75.9, R94.5, K75 or R93.2); viral hepatitis A-E (ICD-10 codes: B15, B15.0, B15.9, B16, B16.0, B16.1, B16.2, B16.9, B17.0, B17.1 or B17.2); other infectious hepatitis (ICD-10 codes: A06.4, B17, B17.8, B25.1, B67.0, B67.5, B67.8, B94.2, O98.4 or P35.3); other liver diseases (ICD-10 codes: K72, K74.1, K75.2, K75.3, K75.4, K75.8, K76, K76.0, K76.1, K76.2, K76.3, K76.4, K76.5, K76.8, K76.9, K77, Q26.6, Q44, Q44.6 or Q44.7); adenovirus infection (ICD-10 codes: A08.2, A85.1, A87.1, B30.0, B30.1, B34.0, B97.0 or J12.0); COVID-19 (ICD-10 codes: B34.2, B97.2, U07.1, U07.2, U09.9, U10.9, U12.9 or U92.1); other viral infection (ICD-10 codes: B25.9, B25, B27.1, B25.8, B25.2, B25.0, A90, A91, D82.3, B30.3, A85.0, B97.1, B08.4, B08.5, A88.0, B34.1, A87.0, A08.1, A95, A95.9, A95.0, A95.1 or Z24.3); and complications of liver transplant (ICD-10 codes: T86.4 or Z94.4).
Causes of hospitalization in children/adolescents (< 18 years-old) in the Brazilian Public Health System from January/2019 to February/2022.
Data expressed as a number (%).Disease categories were classified based on International Classification of Diseases (ICD-10) codes as hepatitis of unknown etiology (ICD-10: B19, B19.0, B19.9, K72.0, K72.9, K75.9, R94.5, K75 or R93.2); viral hepatitis A-E (ICD-10 codes: B15, B15.0, B15.9, B16, B16.0, B16.1, B16.2, B16.9, B17.0, B17.1 or B17.2); other infectious hepatitis (ICD-10 codes: A06.4, B17, B17.8, B25.1, B67.0, B67.5, B67.8, B94.2, O98.4 or P35.3); other liver diseases (ICD-10 codes: K72, K74.1, K75.2, K75.3, K75.4, K75.8, K76, K76.0, K76.1, K76.2, K76.3, K76.4, K76.5, K76.8, K76.9, K77, Q26.6, Q44, Q44.6 or Q44.7); adenovirus infection (ICD-10 codes: A08.2, A85.1, A87.1, B30.0, B30.1, B34.0, B97.0 or J12.0); COVID-19 (ICD-10 codes: B34.2, B97.2, U07.1, U07.2, U09.9, U10.9, U12.9 or U92.1); other viral infection (ICD-10 codes: B25.9, B25, B27.1, B25.8, B25.2, B25.0, A90, A91, D82.3, B30.3, A85.0, B97.1, B08.4, B08.5, A88.0, B34.1, A87.0, A08.1, A95, A95.9, A95.0, A95.1 or Z24.3); and complications of liver transplant (ICD-10 codes: T86.4 or Z94.4).
A total of 1535 children/adolescents [48.2% male sex, 41.6% with age < 6 years, 32.2% in the Southeast region] were hospitalized with an ICD-10 code that represents cases of hepatitis with unknown etiology from January/2019 to February/2022. The top ICD-10 codes reported in those hospitalizations were B19.9 [unspecified viral hepatitis without hepatic coma; 39.9% (n = 612)], K72.9 [hepatic failure, unspecified; 29.8% (n = 457)], and K72.0 [hepatic failure, not elsewhere classified; 14.5% (n = 223)]. The mean (SD) length of hospital stay in those cases was 7.84 (8.36). A total of 8.5% (n = 131) needed a liver transplantation, and 7.0% (n = 107) died during the hospital stay. No significant differences in socio-demographic characteristics, as well as in proportions of ICD-10 codes, liver transplant, or death were observed among the analyzed years (2019–2022) (Table 3).
Characteristics of children/adolescents (< 18 years-old) hospitalized due to suspected hepatitis with unknown etiology from January/2019 to February/2022 in the Brazilian Public Health System.
Data expressed as either number (%) or mean (SD); †p-values obtained either by F-tests (numerical continuous variables) or by chi-square tests (nominal categorical variables). B19=Unspecified viral hepatitis; B19.0=Unspecified viral hepatitis with hepatic coma; B19.9=Unspecified viral hepatitis without hepatic coma; K72=Hepatic failure, not elsewhere classified; K72.9=Hepatic failure, unspecified; K75=Other inflammatory liver diseases; K75.9=Inflammatory liver disease, unspecified; R93.2=Abnormal findings on diagnostic imaging of liver and biliary tract.
The incidence rates of hospitalization for hepatitis with unknown etiology per 1000 all-cause hospitalizations within a year (95% CI) in children aged < 6 years were 6.11 (5.98–6.25), 8.72 (8.52–8.91), and 7.80 (7.63–7.98) in 2019, 2020 and 2021, respectively. In children/adolescents with age from 6 to 11 years, the incidence rates were 12.76 (12.41–13.13), 18.25 (17.73–18.77), and 17.96 (17.46–18.48) per 1000 all-cause hospitalizations, respectively. Additionally, incidence rates of 10.83 (10.56–11.11), 13.23 (12.89–13.57), and 13.28 (12.95–13.62) hospitalizations of adolescents aged between 12 and 17 years per 1000 all-cause hospitalizations were due to hepatitis with unknown etiology in 2019, 2020, and 2021, respectively. Similarly, the incidence rates of hospitalization due to hepatitis with unknown etiology per 1000 all-cause hospitalization (95% CI) in January-February/2022 were 7.52 (7.11–7.94), 16.82 (15.68–18.03), and 13.96 (13.10–14.85) for children/adolescents with age < 6 years, aged between 6 and 11 years, and aged between 12 and 17 years, respectively (Table 4). Fig. 2 shows the incidence rate of hospitalizations (95% CI) due to hepatitis with unknown etiology stratified by age strata from January/2019 to February/2022. No clinically significant trends were observed for changing those incidences in this period (data not shown).
Incidence rates of hospitalization of children/adolescents (< 18 years old) per 1000 all-cause hospitalizations and their 95% confidence interval from January/2019 to February/2022 in the Brazilian Public Health System.
Disease categories were classified based on International Classification of Diseases (ICD-10) codes as hepatitis of unknown etiology (ICD-10: B19, B19.0, B19.9, K72.0, K72.9, K75.9, R94.5, K75 or R93.2); viral hepatitis A-E (ICD-10 codes: B15, B15.0, B15.9, B16, B16.0, B16.1, B16.2, B16.9, B17.0, B17.1 or B17.2); other infectious hepatitis (ICD-10 codes: A06.4, B17, B17.8, B25.1, B67.0, B67.5, B67.8, B94.2, O98.4 or P35.3); other liver diseases (ICD-10 codes: K72, K74.1, K75.2, K75.3, K75.4, K75.8, K76, K76.0, K76.1, K76.2, K76.3, K76.4, K76.5, K76.8, K76.9, K77, Q26.6, Q44, Q44.6 or Q44.7); adenovirus infection (ICD-10 codes: A08.2, A85.1, A87.1, B30.0, B30.1, B34.0, B97.0 or J12.0); COVID-19 (ICD-10 codes: B34.2, B97.2, U07.1, U07.2, U09.9, U10.9, U12.9 or U92.1); other viral infection (ICD-10 codes: B25.9, B25, B27.1, B25.8, B25.2, B25.0, A90, A91, D82.3, B30.3, A85.0, B97.1, B08.4, B08.5, A88.0, B34.1, A87.0, A08.1, A95, A95.9, A95.0, A95.1 or Z24.3); and complications of liver transplant (ICD-10 codes: T86.4 or Z94.4).
This study highlighted that a non-negligible number of cases of hepatitis without a well-recognized etiology has been hospitalized in Brazil in the last years. In May 2022, the Brazilian Ministry of Health (BMoH) established a national algorithm for notification and follow-up of suspected cases of acute hepatitis with unknown etiology in children/adolescents (< 18 years) detected after April/2022. Up to June 29th 2022, a total of 163 cases were registered in 19 federative units. Of them, two (1.2%) were considered as probable and seven (4.3%) as suspected cases of hepatitis of unknown etiology; one subject lost follow-up, 86 remained under investigation, and 67 (41.1%) were rejected, mainly due to liver enzymes lower than 500 UI/L and/or diagnosis of arboviruses. The first probable and suspected cases in Brazil were registered on May 27th 2022 and June 6th 2022, respectively. The main symptoms of cases under investigation were jaundice (67%), fever (54%) and/or digestive symptoms (51%). The median age was six years (range, 4 months-16 years) and the use of few potential hepatotoxic drugs was identified in those cases that remained under investigation, such as paracetamol and amoxicillin. Of all registered cases in Brazil, 6% (n = 10) needed liver transplant and another 6% (n = 10) died.5 Our findings of cases hospitalized with an ICD-10 code considered as hepatitis with unknown etiology in previous years were very similar to those characteristics of suspected cases recently notified by local health authorities to the BMoH.
Characteristics of cases of hepatitis with unknown etiology identified in our analysis in the last years were also aligned to those recently described in international publications. Baker et al. reported nine cases of acute hepatitis with unknown etiology in children under six years in a hospital in Alabama (USA). Most were female (n = 7), none died and two needed a liver transplant.9 As of June 13th 2022, 260 cases (251 confirmed and nine possible) were identified in the UK. Of these, 4.6% (n = 12) needed liver transplant and no children died. While new cases continue to be identified in the UK, an overall decline in the number of new cases reported per week has been observed.10 As of June 30th 2022, a total of 473 cases of acute hepatitis with unknown etiology were reported in 21 countries of the European region. Most cases occurred in children with age < 5 years (76%). Additionally, up to 30% were admitted to an intensive care unit and up to 8% have received liver transplantation.4 Globally, 5% (n = 46/1010) of cases required liver transplant and 2.2% (n = 22/1010) died as the WHO report from July 12th 2022.3
It remains unclear whether there is a real rise in pediatric acute liver failure cases in comparison to previous years, or an increase in awareness of hepatitis cases in children. A web-survey completed by 34 centers in 22 European countries reported that there is no clear overall increase in the occurrence of severe hepatitis in children in the first four months of 2022 compared to data from previous years.11 In our analysis, the incidence rate of hospital admissions per 1000 all-cause hospitalizations, the proportion of liver transplantations and deaths during a hospital stay due to hepatitis with unknown etiology in children/adolescents were similar in the pre-pandemic (2019) period and during subsequent years of the COVID-19 pandemic (Jan 2020-Feb 2022).
Several hypotheses have been raised to explain the causes of those non-A-E hepatitis cases.12 Adenoviruses infections have become one of the most suspected etiologic agents since up to 53% of cases in Europe tested positive.4 This could be explained by an immune deficit in children due to lower exposure to pathogens during COVID-19 associated with a massive wave of adenovirus infections in the last year. Other hypotheses could be prior infection with SARS-CoV-213 or an idiosyncratic drug reaction for treatment of symptoms during viral infections.2 Additionally, a hypothesis of a new hepatotropic virus or new variants of adenovirus or SARS-CoV-2 cannot be discarded.14-16 Probably, there is not a relationship with the COVID-19 vaccine because most cases occurred in children with age < 5 years in whom the COVID-19 vaccine was being administered worldwide. A total of 24% (n = 113/473) of European cases had data on COVID-19 vaccination and 86% (n = 97/113) of them were unvaccinated.4
This study has several limitations. Our analysis was performed in a publicly available anonymous nationwide database of hospitalizations in the Brazilian Public Health System. Therefore, we did not have access to individual medical records, laboratory exams or imaging results to check potential concomitant infectious diseases or use of hepatotoxic drugs. However, the classification of the cause of hospitalization in the SIH-SUS database using ICD-10 codes follow a systematic procedure as previously described by the BMoH (http://tabnet.datasus.gov.br/cgi/sih/mxcid10lm.htm). In our analysis, no children/adolescents hospitalized had a concomitant ICD-10 code of adenovirus or SARS-CoV-2 infection with any of those that defined hepatitis with unknown etiology, respectively. However, we acknowledge that the results of this study might have been biased since our classification for hepatitis of unknown etiology was based on ICD-10 codes registered in the report of hospitalization. We are aware of a lack of standardization for registration of hospitalization cause in a few Brazilian regions. Additionally, we could not access data from March to May 2022 because they were not publicly available at the time of the analysis. Therefore, we could not compare the proportion of cases reported after the outbreak alert by WHO with previous years to evaluate a recent potential increase in cases of hepatitis with unknown etiology. In addition, our analysis is restricted to cases that required hospital admission that might be a sub-sample of cases of hepatitis with unknown etiology. The main strength was the estimation of the burden of hepatitis with unknown etiology using a nationwide database of almost 95,000 hospitalizations of children due to hepatic or infectious diseases with potential liver injury diseases in Brazil. Additionally, hospital admissions data in the Public Health System has been systematically updated by the Brazilian Unified Health System Information Technology Department (DATASUS). Finally, in our analysis, acute hepatitis of unknown etiology was defined by the presence of ICD-10 codes describing liver failure or hepatic inflammation without a specific cause in absence of any other code that could be related to other liver diseases to avoid misclassification of cases.
In conclusion, this study reported a non-negligible number of hospitalizations due to hepatitis with unknown etiology in children/adolescents in the last years in Brazil. Additionally, around 10% of those cases needed liver transplantation or died. Our finding raises the hypothesis that we might be facing a unusual scenario regarding the incidence of hepatitis of unknown etiology in children in Brazil. However, we acknowledge that further prospective studies are needed to confirm these findings. Additionally, high-quality process for the identification of new cases and the further investigation of those recent cases of acute hepatitis in young people remain essential to clarify the etiology and to recommend preventive measures.
List of abbreviationsALT, alanine transaminase; AST, aspartate transaminase; BMoH, Brazilian Ministry of Health; CDC, Center of Disease Control and Prevention; CI, confidence interval; COVID-19, coronavirus 2019 disease; ICD-10, International Classification of Diseases −10; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation; WHO, World Health organisation.
Authors' contributionsRC: study concept and design; interpretation of data; drafting and critical revision of the manuscript; MRA: study concept and design; statistical analysis; interpretation of data; drafting and critical revision of the manuscript; VGV: interpretation of data and critical revision of the manuscript; HP: study concept and design; interpretation of data; drafting and critical revision of the manuscript.
FundingThis study was supported by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro [grant E-26/210.351/2021 for HP], Programa Inova Fiocruz/Fundação Oswaldo Cruz [grants: VPPCB-002-FIO-20–2–11 and VPPIS-005-FIO-20–2–49 for RC; VPPCB-005-FIO-20–2–45 and VPPCB-005-FIO-20–2–61 for MRA]. The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Ethics approval statementThe Brazilian National Hospitalization Registry database (“Sistema de Informações Hospitalares do SUS – SIH/SUS”) from the Brazilian Unified Health System Information Technology Department (DATASUS) is an anonymous, open-source, and web available database (ftp://ftp.datasus.gov.br/dissemin/publicos/SIHSUS).
Data availability statementAll data from the current study were reported in the manuscript, tables, and supplementary material. In addition, data are available upon request to Hugo Perazzo, the corresponding author, from the Evandro Chagas National Institute of Infectious Diseases – Oswaldo Cruz Foundation, Rio de Janeiro (RJ), Brazil.





![Incidence rate per 1000 hospitalizations (95% confidence interval) of hospital admissions due to hepatitis with unknown etiology stratified by age strata [< 6 years; 6–11 years and 12 to 17 years] from January/2019 to February/2022. Incidence rate per 1000 hospitalizations (95% confidence interval) of hospital admissions due to hepatitis with unknown etiology stratified by age strata [< 6 years; 6–11 years and 12 to 17 years] from January/2019 to February/2022.](https://static.elsevier.es/multimedia/14138670/0000002600000006/v1_202212210748/S1413867022004081/v1_202212210748/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w95uaF0+42b+pWE4hY44gaZY=)


