While 20–40% of patients with hepatitis C virus (HCV) monoinfection will spontaneously clear the virus, less is known regarding clearance with coinfections. HCV, human immunodeficiency virus (HIV), and human T-cell lymphotrophic virus 1 and 2 (HTLV-1/2) coinfection occurs due to shared routes of transmission and is prevalent in Brazil.
ObjectivesTo compare the proportion of patients who have spontaneously cleared HCV in patients with HCV monoinfection to patients coinfected by HCV/HIV, or HCV/HIV/HTLV-1.
MethodsUsing medical records from two clinics in Salvador, Brazil, including demographic data and serological markers of HCV, HIV and HTLV-I/II, cross-sectional data was obtained from 197 patients. Patients who were anti-HCV positive and HCV RNA negative, and who did not receive HCV treatment were defined as having cleared infection.
ResultsNineteen patients (9.5%) showed evidence of spontaneous HCV clearance; with clearance in 9 of 108 (8.3%) patients in the HCV monoinfected group, 5 of 68 (7.4%) patients with HCV/HIV, and 5 of 21 (23.8%) patients with HCV/HIV/HTLV. Demographic data were not associated with HCV clearance status. Patients coinfected with both HIV and HTLV-1 had increased odds (5.50; 95% CI 1.00, 30.17) of spontaneous clearance of HCV compared with patients who were HIV negative or of unknown HIV status.
ConclusionOur study found that patients coinfected with HIV and HTLV-1 were more likely to spontaneously clear hepatitis C virus than patients with HIV/HCV or HCV alone. The effects of HTLV coinfection on the immune response of such patients may be associated with these findings.
The World Health Organization estimates that 180 million people, or 3% of the world's population, are infected with hepatitis C virus (HCV) and that 130–170 million have chronic infection.1,2 HCV is tropic for hepatocytes and is a significant cause of morbidity, including cirrhosis and hepatocellular carcinoma, and mortality globally.3 HCV is a blood-borne pathogen, often in coinfection with other blood-borne infections, including human immunodeficiency virus (HIV) and human T-cell lymphotrophic virus-1/2 (HTLV-1/2). This is likely due to shared routes of transmission, such injection drug use (IDU), and in some contexts, contaminated blood products.4,5
Spontaneous resolution of HCV is of both scientific and clinical interest as knowledge of the time-course and factors associated can inform clinical care and prevention technologies, such as vaccine development. There is consistent evidence that about one quarter (25%) of HCV mono-infected individuals spontaneously resolve HCV infection.6,7 Several factors have been shown to be associated with spontaneous HCV clearance, including female sex, non-African ethnicity, jaundice at presentation, HLA type II alleles, and genetic factors, most strongly, IL28B allele variants.6–11 Prospective studies in acutely infected patients have shown that clearance of HCV infection is associated with a strong and broad host immune T-cell response.12–16
Little is known about frequency of spontaneous clearance and disease progression in individuals with triple infection: HCV, HIV and HTLV. HCV clearance in patients coinfected with HIV has been shown to be markedly reduced, with only 10–20% spontaneous clearing.17–19 Furthermore, HIV/HCV coinfected patients have significantly higher HCV viremia levels than in monoinfected patients and reduced response to anti-retroviral therapy.20,21 Much less is known about the natural history of HCV infection in the context of HTLV. HTLV-1 infection can cause adult T-cell leukemia/lymphoma (ATLL), which is a proliferation of CD4+ T-cells, and Th1 activation which is associated with neurological disease.22 HTLV replicates within T-cells and thereby compromises the cell-mediated immune response that may influence HCV clearance.23 Three studies of HCV/HTLV-1 coinfection, all of which were conducted in groups too small to demonstrate differences, have shown inconsistent results with respect to HCV clearance.24–26 Studies in Japan have found that HCV/HTLV-1 coinfected patients have, on average, increased HCV viremia,25 which is consistent with another study showing that coinfection had a multiplicative effect on the development of liver disease.27 In a recent study of patients infected with HCV/HIV/HTLV-1/2 in Brazil, we found that those with triple infection had on average lower ALT levels than patients coinfected with HIV/HCV, suggesting lower hepatic inflammation in the former group.28
Northeastern Brazil is a good location to study coinfection with these three viruses as HTLV-1 and HCV are endemic with a large population of asymptomatic carriers.29,30 HCV has been estimated to infect 1.1% of Brazilians.4 In the general population in Salvador, the prevalence of HTLV-1 and HIV is estimated at 1.35% and 0.55%, respectively.29,31 Data from very select populations in Brazil, including IDU, have found high infection rates for HCV, HTLV-1 and HIV: 70%, 22% and 44%, respectively.31,32 In this region, 80% of the population is of African descent,33 providing unique population in which to study spontaneous clearance, particularly as several studies have shown that black and racially diverse populations have lower HCV clearance rates.9,25,34
ObjectivesTo address the paucity of data on the impact of co-infection and triple infection with HCV, HIV and HTLV, we conducted a cross-sectional chart review. We compared the rates of HCV clearance in a cohort of mono- and co-infected patients with HCV, HCV/HIV and HCV/HIV/HTLV from two referral centers in Salvador, Brazil.
Study designStudy populationCross-sectional data were abstracted from medical charts of patients infected with HCV, HIV/HCV, and HIV/HTLV/HCV in two outpatients services in Salvador, Bahia, Brazil: Hospital Universitario Professor Edgard Santos of Federal University of Bahia (HUPES) and Centro Estadual de Diagnostico, Assistencia e Pesquisa (CEDAP), the central HIV/AIDS Referral Center in the state of Bahia. HUPES and CEDAP are both public institutions that serve patients infected with HIV and viral hepatitis in Bahia. Both CEDAP and the infectious disease outpatient clinic at HUPES see approximately 10,000 patients a year. Patients from CEDAP were primarily coinfected with HIV (including those with HCV, or triply infected with HCV, HIV and HTLV-1). HUPES patients were primarily HCV mono-infected, or HCV/HTLV coinfected.
Data collectionPatients’ records from 2008 to 2010 were reviewed; all patients who were anti-HCV positive and aged 18 or older were included. Sociodemographic data collected included age, sex, race, and where medical care was received. Age was categorized by five-year intervals. Race categories were those used in the medical charts, either white, meaning of Caucasian descent, black, of African descent, or “pardo”, racially mixed descent. All data were abstracted twice by different research assistants, and then double entered into an EPIinfo 3.5.4 database (CDC, GA, USA). Patients were grouped as anti-HIV negative or unknown, anti-HIV positive and anti-HTLV negative or unknown and, finally, anti-HIV positive and anti-HTLV positive.
Laboratory testsLaboratory tests results were obtained from medical records, including anti-HCV and HCV RNA, as well as HIV and HTLV results. The majority of laboratory testing was conducted in laboratories at the respective clinic or hospital where patients were seen. In brief, testing was done as follows: anti-HCV was tested for using enzyme immunoassay (EIA) (different manufacturers, but most commonly Elisa Meia Axsym tests (Abbott ELISA Kit, USA); HIV testing was determined using two enzyme immunoassays (EIA) (HIV-1 QT, Bio Manguinhos, Rapid Check HIV 1 and 2) and confirmed by western blot (Genelabs, Singapore). HTLV-1 was tested by EIA (Abbott Biomerix Kit; USA), and confirmed by western blot (Genelabs, Singapore). HCV RNA was tested for using HCV Amplicor 2.0; Roche Diagnostics, Pleasenton, CA) with lower limit of detection of 50UI/mL (120cp/mL). Results negative for HCV RNA were classified as being aviremic.
Statistical analysesThe main outcome variable of our study was HCV clearance. Patients who were anti-HCV positive and HCV RNA negative, and with no record of HCV treatment were classified as having spontaneously cleared HCV. Patients who were HCV RNA positive or who received treatment were categorized as having HCV infection. Covariates of interest were co-infection with HIV and/or HTLV-1. Participants were divided into three groups: patients with negative or unknown HIV status and negative or unknown HTLV status (Group 1), patients with positive HIV status and negative or unknown HTLV status (Group 2) and, finally, patients with positive HIV and HTLV-1 status (Group 3). There were no patients with positive HTLV status whose HIV status was unknown. Univariate associations between groups by sociodemographic and clinical factors were assessed using Fisher's exact tests. Firth's logistic regression was used to assess factors independently associated with HCV clearance by coinfection status, adjusting for potential confounders including age, site and sex. Odds ratios (OR), adjusted odds ratios (AOR) and 95% confidence intervals (CI), were estimated. Race was excluded from multivariate analyses due to the significant amount of missing data and large number of participants who were classified as mixed race. All analyses were conducted using STATA for Mac statistic software package version 11.0 and the Firth logit module with distribution date: 2008/07/14.
Ethical reviewThe study protocol was reviewed and approved by the Research Ethics Committee of the Secretary of Health of the State of Bahia (Comite de Etica em Pesquisa da Secretaria de Saude do Estado da Bahia – CEP SESAB) and the UCSF Committee on Human Research. Informed consent for this retrospective chart review was waived.
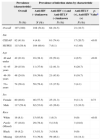
ResultsThe study team screened 575 patients for inclusion in the study; 357 participants were excluded because they were not anti-HCV positive. Medical charts from 218 patients were reviewed, of which 21 were excluded due to missing HCV RNA status, leaving 197 records for analyses. Over half of the patients (n=115, 58.4%) received care at HUPES, the remaining at CEDAP. The majority of patients were male (n=117 or 59.4%); mean age was 48.5 years (SD 9.67). Information on race/ethnicity was available for 69 patients, among those, 23.2% were White, 53.6% as “Pardo” and 23.2% as Black (Table 1). Overall, 9.5% (n=19) patients had evidence of spontaneous HCV clearance. There were significant differences between the HIV/HTLV groupings by clinical site, principally since the infectious disease clinic at HUPES saw few HIV infected patients. HCV monoinfected patients were significantly older than co-infected patients with a majority (78.4%) over age 50.
Demographic characteristics of HCV-positive participants overall and by HIV and HTLV status at two hospitals in Salvador, Brazil.
| Prevalence (characteristic) | Prevalence of infection status by characteristic | ||||
|---|---|---|---|---|---|
| Overall | Anti-HIV (−)/unknown | Anti-HIV (+) and Anti-HTLV (−)/unknown | Anti-HTLV (+), Anti-HIV (+) | p-Valuea | |
| N (%) | N (%) | N (%) | N (%) | ||
| Overall | 197 (100) | 108 (54.8) | 68 (34.5) | 21 (10.7) | |
| Site | |||||
| CEDAP | 82 (41.6) | 4 (4.8) | 61 (74.4) | 17 (20.7) | <0.01 |
| HUPES | 115 (58.4) | 104 (90.4) | 7 (6.1) | 4 (3.48) | |
| Age | |||||
| 40 and under | 42 (21.8) | 16 (38.1) | 22 (52.4) | 4 (9.5) | <0.01 |
| 41–45 years | 29 (15.0) | 11 (37.9) | 12 (41.3) | 6 (20.7) | |
| 46–50 years | 48 (24.9) | 19 (39.6) | 21 (43.8) | 8 (16.7) | |
| 51+ years | 74 (38.4) | 58 (78.4) | 13 (17.6) | 3 (4.1) | |
| Sex | |||||
| Female | 80 (40.6) | 46 (57.5) | 25 (31.3) | 9 (11.3) | 0.73 |
| Male | 117 (59.4) | 62 (53.0) | 43 (36.8) | 12 (10.3) | |
| Race | |||||
| White | 16 (8.1) | 15 (93.8) | 1 (6.3) | 0 (0) | <0.01 |
| Pardo (Mixed) | 37 (18.8) | 29 (78.4) | 5 (13.6) | 3 (18.6) | |
| Black | 16 (8.2) | 13 (81.3) | 3 (18.8) | 0 (0) | |
| Missing | 128 (65.0) | 51 (39.8) | 59 (46.1) | 18 (14.1) | |
Table 2 shows unadjusted and adjusted associations between co-infections and demographic characteristics with HCV clearance status. HIV co-infection was not associated with HCV clearance. However, positive HTLV status was consistently associated with over three-fold higher odds of HCV clearance in a univariate and multivariate models. As well, the proportion of HIV/HTLV-1 positive patients that spontaneously cleared HCV was significantly higher compared to HIV-negative patients with adjusted odds of HCV clearance of 5.50 (95% CI 1.00–30.17, p=0.049).
Bivariate and multivariate associations of HIV/HTLV co-infection status and demographic characteristics with spontaneous HCV clearance in patients at two hospitals in Salvador, Brazil.
| HCV spontaneous clearancen/N (col%) | OR (95% CI) | p-Value | Adjusted ORa (95% CI) | p-Value | |
|---|---|---|---|---|---|
| Coinfection status | |||||
| HIV−/unknown | 9/108 (8.3) | 1 | 1 | ||
| HIV+ and HTLV−/unknown | 5/68 (7.4) | 0.91 (0.30, 2.71) | 0.86 | 1.61 (0.29, 8.97) | 0.58 |
| HIV+ and HTLV+ | 5/21 (23.8) | 3.49 (1.08, 11.27) | 0.037 | 5.50 (1.00, 30.17) | 0.049 |
| HIV | |||||
| Negative | 2/44 (4.6) | 1 | |||
| Positive | 10/89 (11.2) | 2.25 (0.54, 9.37) | 0.27 | ||
| Unknown | 7/64 (10.9) | 2.22 (0.50, 9.79) | 0.29 | ||
| HTLV | |||||
| Negative | 5/76 (6.6) | 1 | 1 | ||
| Positive | 5/24 (20.8) | 3.67 (1.02, 13.2) | 0.047 | 3.58 (1.00,12.90) | 0.051 |
| Unknown | 9/97 (9.3) | 1.40 (0.47, 4.17) | 0.56 | 1.24 (0.22, 4.64) | 0.75 |
| Age | |||||
| 40 and under | 4/42 (9.5) | 1 | |||
| 41–45 years | 4/29 (13.8) | 1.51 (0.37, 6.12) | 0.56 | ||
| 46–50 years | 4/48 (8.3) | 0.87 (0.22, 3.43) | 0.84 | ||
| 51–80 years | 6/74 (8.1) | 0.81 (0.23, 2.88) | 0.75 | ||
| Sex | |||||
| Female | 9/80 (11.3) | 1 | |||
| Male | 11/117 (8.6) | 0.74 (0.29, 1.86) | 0.52 | ||
| Race | |||||
| Branco (White) | 1/16 (6.3) | 1 | |||
| Pardo (Mixed) | 1/37 (2.7) | 0.42 (0.04, 4.41) | 0.47 | ||
| Negro (Black) | 3/16 (18.8) | 2.68 (0.35, 20.75) | 0.35 | ||
| Missing | 14/128 (10.9) | 1.31 (0.22, 7.63) | 0.77 | ||
| Site | |||||
| CEDAP | 8/82 (9.7) | 1 | |||
| HUPES | 11/115 (9.6) | 0.96 (0.38, 2.46) | 0.94 | ||
In this study of HCV infected patients in Salvador, Bahia, Brazil, we found that patients who were coinfected with HTLV had greater likelihood of spontaneous clearance of HCV infection overall. Additionally, those with HIV and HTLV infection had an increased clearance: almost a quarter (23.9%) of patients who had evidence of HCV infection and who were also coinfected with the two other viruses demonstrated evidence of spontaneous clearance compared to 8% of HIV-positive/HTLV-negative patients. Our results suggest the possibility that HTLV infection may have immune effects that enhance responses to HCV infection. In a previous study we showed that cultured mononuclear cells from these HIV/HTLV coinfected patients had a higher spontaneous production of IL-1, γ-interferon, and lower production of IL-4.35 In addition, in a recent work, we also detected significantly higher serum levels of eight proinflammatory cytokines (IL-1b, IL-2, FGF, γ-IFN, IP-10, MIP-1 α, MIP-1 β, TNF-α) in patients coinfected with HIV-HCV and HTLV, in comparison to HIV-HCV coinfected individuals.36 There was also a strong association between higher levels of these cytokines and sustained virological response, among patients treated for HCV infection.36 We do not know the temporal order in which patients were infected, however if HTLV infected patients develop higher HCV viremia in acute infection, as suggested by other studies,25 this could also be associated with immune responses that increase HCV clearance.37,38 Taken together, these results suggest that this profile of immune response is a likely result of HTLV coinfection, potentially contributing to the higher rate of spontaneous HCV clearance seen in patients in this study. We believe these new data can point to new directions for immunology studies of successful HCV infection responses. A better understanding of such mechanisms could be translated in future improvements on treatment for patients coinfected with HIV and HCV.
Our study had many limitations including a small sample size that restricted our statistical power resulting in large confidence intervals with results of nominal significance. We chose to use Firth logistic regression in order to adjust for important covariates, such as sex, and due to our small sample size with separation. We also had a significant amount of missing data that we were unable to obtain from the medical charts, particularly in regards to race, as well as coinfections. In regard to the missing data for race, the data that was collected reflected the racial distribution found in other studies conducted in the state of Bahia as well as census data.39–41 Given the large amount of missing data and that the majority of participants were of mixed racial background, this variable did not add meaningful information to our multivariate model. With respect to missing coinfection data, we conducted further sensitivity analyses examining less conservative groupings assuming patients with unknown HIV status or HTLV status were positive for HIV and HTLV. These analyses also showed similar significant findings: patients with HIV and HTLV had increased odds of spontaneous clearance of HCV. We used two different clinics to obtain our study population, which could also have introduced selection or information bias if clinical data was collected with systematic differences, which we could not assess. We did not identify if patients were infected with HTLV-1 or II; however HTLV-1 is present in 95% to 98% of all infections in Salvador, so it is likely the prevalent infection in this analysis.39 We were also not able to assess genetic associations between interferon-lambda 3 (IFNL3, previously called IL28B), and which is known to be associated with HCV clearance.42,43 Our sampling allowed us to capture the majority of patients with recent HCV infections receiving care in the state of Bahia; however, our study has inherent selection bias as HIV infected patients received more comprehensive and regular testing for HCV than non-HIV infected patients. Finally, this was a cross-sectional study using historical data, which limits our ability to fully control for all confounders and determine causality.
Very little has been published on the role of coinfections in patients with HCV. Despite its limitations, our study is the first to examine spontaneous HCV clearance in patients infected with HTVL and HIV, a more complex and potentially realistic scenario than examining these diseases individually as many of these infections coexist and are transmitted via similar routes. The present findings suggest that HTLV coinfection may have a positive effect on the spontaneous clearance of HCV, especially in HIV-1 coinfected patients. The available research on the immune response in this population indicates that the production of proinflammatory cytokines is upregulated in coinfected individuals, potentially contributing to HCV clearance. Taken together, these results provide a new insight on the role of specific immune response in HCV infection and advance understanding of HTLV and HCV infections.
FundingNone.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to acknowledge Alexandre Azevedo Ziomkowski, Adriana Fonseca Oliveira de Melo, Tassia Maria Oliveira dos Santos for their invaluable help with data collection.