Questionnaire and spirometry were applied to post-tuberculosis indigenous and non-indigenous individuals from Dourados, Brazil, to investigate the prevalence of chronic respiratory symptoms and pulmonary dysfunction.
MethodsThis was a cross-sectional study in cured tuberculosis individuals as reported in the National System on Reportable Diseases (SINAN) from 2002 to 2012.
ResultsOne hundred and twenty individuals were included in the study and the prevalence of chronic respiratory symptoms was 45% (95% CI, 34–59%). Respiratory symptoms included cough (28%), sputum (23%), wheezing (22%) and dyspnea (8%). These symptoms were associated with alcoholism, AOR: 3.1 (1.2–8.4); less than 4 years of schooling, AOR: 5.0 (1.4–17.7); and previous pulmonary diseases, AOR: 5.4 (1.7–17.3). Forty-one percent (95% CI, 29–56) had pulmonary disorders, of which the most prevalent were obstructive disorders (49%), followed by obstructive disorder with reduced forced vital capacity disorders (46%) and restrictive disorders (5%). The lifestyle difference could not explain differences in chronic symptoms and/or the prevalence of pulmonary dysfunction.
ConclusionThe high prevalence of chronic respiratory symptoms and pulmonary dysfunction in post-tuberculosis patients indicates a need for further interventions to reduce social vulnerability of patients successfully treated for tuberculosis.
Tuberculosis (TB) is a chronic disease with one of the highest morbidity and mortality rates worldwide. Certain groups, such as indigenous populations, may be more susceptible to developing the disease.1–3 The incidence of TB among indigenous people is consistently higher than in the general population. Between January 2002 and December 2008, the mean of annual TB notifications in the indigenous population of Dourados was 260 per 100,000 inhabitants compared to only 25 per 100,000 inhabitants in non-indigenous populations.4–6 Among treated and cured TB patients, some may develop respiratory sequelae characterized by chronic respiratory symptoms, including cough, sputum, and dyspnea. These sequelae may persist even in individuals who have been properly treated for TB and should not be overlooked as they have a negative impact on the individual's quality of life.7
There is no consensus on which disorder is the most prevalent in individuals with TB sequelae.7–12 Population-based studies are needed to investigate the persistence of chronic symptoms and changes in lung function. In addition, indigenous populations have different immune responses and risk factors associated with TB compared with non-indigenous populations.13,14 Thus, further studies are needed to clarify whether there are differences with regard to the prevalence of these changes between these two populations. In this sense, the objective of this study was to investigate the prevalence of chronic respiratory symptoms and pulmonary dysfunction in post-tuberculosis individuals and to compare these results between indigenous and non-indigenous populations of Dourados-MS.
Material and methodsStudy design and inclusion and exclusion criteriaThis was a cross-sectional population-based study of indigenous and non-indigenous individuals with a history of TB as reported by the National System on Reportable Diseases (SINAN) from January 2002 to December 2012 in Dourados-MS. We included individuals with notifications of TB to the SINAN diagnosed between 2002 and 2012. We excluded individuals under 18 or over 65 years of age, prisoners, residents of other municipalities, and patients with changes in diagnosis or with neurological disorders.
Data collection was conducted by visiting each participant's home from November 2013 to October 2014. The questionnaire was administered to the participants in order to collect sociodemographic, clinical and epidemiological variables that could be associated with the development of pulmonary changes post-tuberculosis such as persistence of respiratory symptoms and pulmonary function. The following variables were considered: gender, age, educational level, nationality, race, occupation, marital status, alcohol use, smoking, passive smoking, previous pulmonary diseases (pulmonary emphysema, bronchitis, and pleural effusion), work in a dusty and/or smoky environment, wood-stove use, and persistence of respiratory symptoms such as cough, phlegm, sputum, wheezing, and dyspnea after successful TB treatment.
SpirometryEvaluations of pulmonary function were performed by spirometry using a portable spirometer Koko Spirometer (manufactured by nSpire Health, Inc, Lefthand Circle, Longmont, USA, Koko PFT Software, Series No. 1329K3A39) that allowed for the new Brazilian standards for calculating the theoretical value of adults according to the new reference values for forced spirometry in Brazilian populations to be used.12 We evaluated the forced expiratory volume in one second (FEV1), the forced vital capacity (FVC), ratio of the forced expiratory volume in one second to the forced vital capacity (FEV1/FVC), and the forced expiratory flow between 25 and 75% (FEF25–75%). The tests consisted of pre- and post-bronchodilator phases, the latter obtained 15min after the administration of 400μg of salbutamol.
Patients were classified in accordance to the Guidelines for Pulmonary Function Tests of the Brazilian Society of Pneumology and Tisiology. Spirometry was considered as normal when the FVC, FEV1 and FEV1/FVC were equal to or greater than 80% of the predicted value. Obstructive disorder was considered when the FEV1/FVC ratio was below 80% and FEV1 was less than 80% of the predicted value. A patient was classified with a restrictive disorder when the FEV1/FVC ratio was less than 80% and FVC was below 80% of the predicted value. Obstructive disorder with reduced forced vital capacity was considered when the difference between FVC and FEV1 for the pre-bronchodilator phase was less than or equal to 12%.15
Statistical analysisAll clinical data were entered in duplicate into the electronic database EpiData, version 3.1 (The EpiData Association, Odense, Denmark), and SAS version 9.2 (SAS Institute, Cary, NC) was used to analyze the univariate and multivariate models associated with chronic symptoms. Dichotomized and categorical data were analyzed with the chi-squared test or Fisher's exact test. For continuous variables, the t-test or analysis of variance (ANOVA) were utilized. Univariate analyses were performed to verify the associations between the dependent and independent variables, and those achieving a pre-specified level of significance (p<0.20) were included in the multivariate analysis. Logistic regression analysis was used to estimate the adjusted odds ratios.
Ethical considerationsAll eligible individuals were informed about the study, and the questionnaire and spirometry were performed after receiving a written approval in the informed consent. Informed consent forms in the Guaraní language were used for the indigenous population. The consent forms were read to the illiterate participants and they provided their consent using their fingerprint. The project was approved by the Research Ethics Committee of the Federal University of Grande Dourados and by the National Research Ethics Committee of the National Health Council (CAAE: 05532912.8.0000.5160/Number: 193.877).
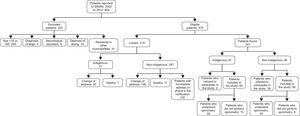
ResultsDuring the study period, 800 individuals were diagnosed with tuberculosis and reported to the SINAN. Of those, 325 were excluded (Fig. 1). We were not able to interview 318 of the 475 individuals included in this study because 166 changed their home address, 18 died, and 130 could not be found due to incorrect address and phone contact information. We contacted 161 individuals, and 25% refused to participate in the study. The final sample of 121 participants was divided into two groups: indigenous (n=61) and non-indigenous (n=60) (Fig. 1). Twenty-one individuals did not reproduce acceptable spirometry curves, including two who presented nausea, four due to missing teeth, 10 failed to perform the maneuvers because of difficulties in understanding the commands, and five were unable to undergo spirometry due to severe dyspnea.
Table 1 shows the sociodemographic characteristics of the indigenous and non-indigenous individuals with a history of tuberculosis. Among the indigenous participants, 57% were male, and 87% had less than four years of schooling. In the non-indigenous group, 47% were male, and 78% had less than four years of schooling; this same group also included more individuals who used alcohol, illicit drugs, and smoked. Non-indigenous individuals had more current history of sputum production than indigenous (p=0.05). Cough, wheezing and dyspnea, as well as the spirometric values, were similar between the ethnic groups considered (Table 1).
Sociodemographic characteristics, chronic respiratory symptoms and spirometry parameters of indigenous and non-indigenous individuals with a history of tuberculosis in Dourados (n=121).
| Variables | RaceNumber (percentage) | p value | |
|---|---|---|---|
| Indigenous (n=61) | Non-indigenous (n=60) | ||
| Sex, male | 35 (57) | 28 (47) | 0.24 |
| Age, years, mean±SDa | 38±15 | 42±15 | 0.20 |
| Marital status, single | 9 (15) | 31 (52) | <0.01 |
| Less than 4 years of schooling | 53 (87) | 47 (78) | 0.21 |
| BMI, mean±SDa | 25±4 | 24±5 | 0.27 |
| Current smoker | 13 (21) | 30 (50) | <0.01 |
| Passive smoker | 6 (10) | 13 (22) | 0.07 |
| Drug use | 1 (2) | 12 (25) | <0.01 |
| Alcoholism | 6 (10) | 18 (30) | <0.01 |
| Work in dusty environment | 9 (13) | 31 (52) | <0.01 |
| Work in smoky environment | 7 (12) | 6 (10) | 0.74 |
| Previous pulmonary diseasesb | 5 (8) | 14 (24) | 0.02 |
| Symptoms | 24 (20) | 31 (26) | 0.17 |
| Cough | 18 (15) | 24 (20) | 0.23 |
| Sputum production | 14 (12) | 24 (20) | 0.05 |
| Wheezing | 14 (12) | 17 (14) | 0.50 |
| Dyspnea grade 2 | 1(1) | 5 (5) | 0.11c |
| Dyspnea grade 3 | 3 (3) | 0 (0) | 0.24c |
| Dyspnea grade 4 | 0 (0) | 1 (1) | 0.49c |
| Spirometry parameters | |||
| FEV1/FVC×100 | 86% | 89% | 0.40 |
| FEV1 | 85% | 87% | 0.62 |
| FVC | 89% | 86% | 0.67 |
| FEF25–75% | 82% | 80% | 0.57 |
| Pattern of spirometry | 0.35 | ||
| Normal | 29 (58) | 30 (60) | |
| Obstructive | 8 (16) | 12 (24) | |
| Obstructive disorder with reduced forced vital capacity | 11 (22) | 8 (16) | |
| Restrictive | 2 (4) | 0 (0) | |
BMI, Body Mass Index; FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; FEV1/FVC, ratio of forced expiratory volume in the first second and forced vital capacity; FEF 25–75%, forced expiratory flow between 25 and 75%.
The prevalence of respiratory symptoms was 45% (95% CI, 34–59%) and included cough (28%), sputum (23%), wheezing (22%), and dyspnea (8%). Compared with asymptomatic individuals, post-tuberculosis individuals with symptoms were more likely to have less then four years of schooling (93% versus 74%, p<0.01), work in dusty environment (44% versus 25%, p=0.03), and to have had previous pulmonary diseases (25% versus 8%, p<0.01) The majority of the individuals who had symptoms had abnormal spirometry when compared to individuals with no symptoms (65% versus 35%, p<0.01) (Table 2). In the multivariate model, the following variables were associated with the presence of symptoms: less than four years of schooling, AOR: 5.0 (1.4–17.7); alcohol abuse, AOR: 3.1 (1.2–8.3); and previous pulmonary diseases, AOR: 5.4 (1.7–17.4) (Table 3). Among the participants, 41% (95% CI, 29–56) had pulmonary disorders, of which the most prevalent were obstructive disorders (49%), followed by obstructive disorder with reduced forced vital capacity disorders (46%), and restrictive disorders (5%). Eighteen individuals had moderate pulmonary dysfunction, and only 1 (6%) had been diagnosed with lung disease and were under medical care.
Differences among patients with a history of tuberculosis stratified by presence of symptoms in Dourados (n=121).
| Variables | SymptomsNumber (percentage) | p value | |
|---|---|---|---|
| Symptoms(n=55) | Non-symptoms(n=66) | ||
| Clinical and epidemiological | |||
| Sex, male | 22 (40) | 41 (62) | 0.01 |
| Race, indigenous | 24 (44) | 31 (56) | 0.17 |
| Marital Status, single | 20 (37) | 21 (32) | 0.53 |
| Age, years, mean±SDa | 43±14 | 38±15 | 0.11 |
| Less than 4 years of schooling | 51 (93) | 49 (74) | <0.01 |
| Current smoker | 22 (40) | 21 (32) | 0.35 |
| Passive smoker | 9 (17) | 10 (15) | 0.85 |
| Drug use | 10 (18) | 6 (9) | 0.14 |
| Alcoholism | 15 (27) | 9 (14) | 0.06 |
| Work in dusty environment | 24 (44) | 16 (25) | 0.03 |
| Work in smoky environment | 8 (15) | 5 (8) | 0.24 |
| Cook with a wood stove | 16 (30) | 15 (24) | 0.48 |
| Previous pulmonary diseasesb | 14 (25) | 5 (8) | <0.01 |
| Pattern of spirometry | <0.01 | ||
| Normal | 14 (35) | 45 (75) | |
| Obstructive | 13 (33) | 7 (12) | |
| Obstructive disorder with reduced forced vital capacity | 11 (27) | 8 (13) | |
| Restrictive | 2 (5) | 0 (0) | |
Risk factors associated with respiratory symptoms in individuals with a history of tuberculosis (n=121).
| Variables | Crude OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| Race, indigenous | 1.64 (0.80–3.39) | |
| Age, per year | 0.98 (0.95–1.00) | |
| BMI | 0.96 (0.88–1.05) | |
| Sex, male | 2.46 (1.18–5.12) | |
| Marital status, single | 1.22 (0.57–2.60) | |
| Less than 4 year of schooling | 4.42 (1.39–14.07) | 5.01 (1.42–17.66) |
| Smoking | 1.42 (0.68–3.02) | |
| Passive smoking | 1.09 (0.41–2.92) | |
| Drug use | 2.22 (0.75–6.56) | |
| Alcoholism | 2.37 (0.94–5.95) | 3.10 (1.16–8.30) |
| Previous pulmonary diseasesa | 4.09 (1.37–12.25) | 5.42 (1.69–17.34) |
| Work in dusty environment | 2.37 (1.09–5.15) | |
| Work in smoky environment | 2.01 (0.61–6.58) | |
| Cook with a wood stove | 1.34 (0.59–3.06) |
BMI, Body Mass Index; OR, odds ratio.
Few studies have evaluated the permanence of post-tuberculosis respiratory symptoms, which reinforces the importance of this research, considering the negative impact and influence on the quality of life of individuals affected by these changes in lung function.7,9,10,16
In our population-based study, we observed the presence of chronic symptoms such as cough, sputum, dyspnea, and wheezing in 45% of subjects even after the completion of treatment and cure of TB. This persistence of respiratory symptoms was also found in a study of adult patients treated in the outpatient TB clinic of a university hospital in which 72% of the 56 patients had chronic symptoms similar to those found in this study.9
Among the prevalent post-tuberculosis symptoms, cough and sputum have been reported, and according to a study in the Institute of Pulmonology, these symptoms were present in 80% of patients followed. Dyspnea was also mentioned by 45% of the patients.17 Sputum production has been correlated with the residual lung damage, as reported by Hnizdo et al.18 Even in individuals who properly treated for tuberculosis, there is persistence of symptoms; recent research has shown that 3/5 of the population studied showed persistent post-tuberculosis respiratory symptoms.7
Delays in the diagnosis and initiation of TB treatment can lead to increased injury to the lung parenchyma, and this damage worsens with the duration of disease, causing persistent respiratory symptoms and lung dysfunction.11,18–20 The results of this study indicate that some factors can be critical to the presence of chronic respiratory symptoms in individuals with a history of tuberculosis; among these factors, there is an emphasis on education, alcohol use, and previous respiratory diseases.2,21 Low level of education is related to low socioeconomic status, and this is a recognized risk factor for TB. In addition, the social condition of the individual can lead to less access to health services and, consequently, to a diagnosis of tuberculosis. Alcohol consumption can also be associated with a late diagnosis, and socioeconomic conditions in both delayed situations expose the individual to longer disease duration and, therefore, an increased likelihood of pulmonary sequelae.2,21
Using diagnostic services, a study in Dourados-MS noted that 75% of indigenous and 65% of non-indigenous individuals sought treatment at the onset of symptoms. However, most patients reported a diagnostic delay; 46% of indigenous and 44% of non-indigenous patients need at least three (3) medical consultations to receive a diagnosis of TB, which took more than five weeks. Therefore, these patients spent more time exposed to the disease and were more susceptible to post-pulmonary tuberculosis sequelae.21
Another study involving an indigenous population of Dourados-MS concluded that this population had greater access to diagnostic tests and treatment compared with the non-indigenous population. The diagnosis and consequent early treatment in the indigenous population compared with the non-indigenous population might explain why there were no differences in the length of symptoms or changes in pulmonary function between the populations studied, even in the face of the different living habits, cultural barriers, and the lower level of education and socioeconomic status of the indigenous population.5,21
Among the individuals who underwent spirometry in our study, 41% had pulmonary function changes: the most prevalent were obstructive disorders (49%), followed by obstructive disorder with reduced forced vital capacity (46%) and restrictive disorders (5%). It remains unclear which respiratory disorder is most prevalent in post-tuberculosis sequelae due to the small number of studies conducted. Ramos et al., Santa Cruz et al. and Di Naso et al. in their respective studies evaluated the association between TB and altered pulmonary function, and there was no common consensus on which disorder was the most prevalent as a sequel.9,19,20
Our study had some limitations, such as a number of refusals to participate by individuals who thought they were cured and had no symptoms and the difficulty of finding the households due to the large number of records with nonexistent phone numbers and addresses. Furthermore, 8% of the indigenous and 24% of the non-indigenous individuals had previous pulmonary diseases and therefore could have symptoms and functional changes attributed to the underlying disease and not related to TB sequelae. Not all patients underwent chest X-ray because the exam was not included in the study objectives.
It is important to emphasize that 17% of the individuals included in the study were unable to complete the spirometry due to difficulty in understanding the guidelines, dental issues and/or chronic respiratory symptoms. Among patients who did not complete the survey, 66% had a low level of education and low socioeconomic status, which most likely hindered their comprehension of the guidelines for performing the required expiratory maneuvers. Most of these individuals were indigenous, and in our opinion, they need further attention regarding their symptoms for the diagnosis of possible pulmonary sequelae.
For the individuals who could not perform the spirometry test, possible pulmonary sequelae could be diagnosed by the respiratory symptoms presented by these individuals. According to Global Obstructive Lung Disease (GOLD, 2014), chronic symptoms should be considered, as they may indicate the severity of pulmonary sequelae.22
The high prevalence of chronic respiratory symptoms and pulmonary dysfunction in post-tuberculosis patients indicates a need for further interventions to reduce social vulnerability in patients with post-tuberculosis.
Conflicts of interestThe authors declare no conflicts of interest.
The authors are grateful to the Special Secretariat of Indigenous Health (Secretaria de Saúde Indígena; SESAI), the Council of Indigenous Health (Conselho Distrital de Saúde Indígena; CONDISI) and the Department of Health at Dourados and the District for their full support during the study period. We thank the indigenous and non-indigenous participants, without whom this study could not have been performed. This work was supported by Brazilian National Research Council (CNPq, N° 404158/2012-9).