Rabies virus (RABV) isolated from different mammals seems to have unique characteristics that influence the outcome of infection. RABV circulates in nature and is maintained by reservoirs that are responsible for the persistence of the disease for almost 4000 years. Considering the different pattern of pathogenicity of RABV strains in naturally and experimentally infected animals, the aim of this study was to analyze the characteristics of RABV variants isolated from the main Brazilian reservoirs, being related to a dog (variant 2), Desmodus rotundus (variant 3), crab eating fox, marmoset, and Myotis spp. Viral replication in brain tissue of experimentally infected mouse was evaluated by two laboratory techniques and the results were compared to clinical evolution from five RABV variants. The presence of the RABV was investigated in brain samples by fluorescent antibody test (FAT) and real time polymerase chain reaction (qRT-PCR) for quantification of rabies virus nucleoprotein gene (N gene). Virus replication is not correlated with clinical signs and evolution. The pattern of FAT is associated with RABV replication levels. Virus isolates from crab eating fox and marmoset had a longer evolution period and higher survival rate suggesting that the evolution period may contribute to the outcome. RABV virus variants had independent characteristics that determine the clinical evolution and survival of the infected mice.
Rabies is an acute viral disease that affects humans and other mammals causing encephalitis or meningoencephalitis. The etiological agent belongs to the Mononegavirale order, the Rhabdoviridae family and the Lyssavirus genus.1 Currently, the International Committee on Taxonomy of Viruses recognizes 12 Lyssavirus species. However, the Rabies Virus (RABV) genotype 1 is the responsible for the vast majority of human rabies cases in the world.2
The main reservoirs of the virus in nature are members of the Carnivora and Chiroptera orders. Classic RABV is maintained by terrestrial mammals worldwide, except in Australia, Antarctica, and several islands, and by bats in the New World only.1 In Brazil rabies is still endemic and independent cycles have been identified in a range of species, such as marmosets (Callithrix jacchus),3,4 in many wild carnivores, crab eating fox (Cerdocyon thous), Pseudalopex vetulus,5,6Dusicyon vetulus,7 and many bat species.8
Rabies is almost an invariably fatal disease, having the highest case fatality rate of any currently recognized infectious diseases.2,9 The disease is associated with intense viral replication in the central nervous system (CNS) which induces the formation of cytoplasmic inclusion bodies after the replication, called Negri Bodies.10 However, recovery has been reported in a few patients, most of whom were infected with bat RABV variants.11 In 2012 five human deaths were registered in Brazil being three of them associated with wildlife species and two due to canine aggression, showing that in Brazil canine rabies still occur in a endemic form, mainly in the northern region.12
Most human deaths in the United States can be attributed to unrecognized exposures to rabies viruses associated mainly with two bat species, Lasionycteris noctivagans and Pipistrellus subflavus. Variants associated with these species account for approximately 70% of rabies deaths and an increased viral infectivity is being associated with those events.13 Normally interspecies infection produces a single fatal spillover and secondary transmission has seldom been observed in nature.14
RABV has unique characteristics that influence the outcome after animal infection. Independent cycles are the result of virus adaptation to replicate preferentially in certain host species.15 Successful completion of viral cycle depends on multiple functions of the RABV, i.e. host cell response modulation and the role of individual viral proteins in infection, which all together define typical pathogenicity and virulence.16
Clinical presentation may be variable, even in patients affected by lyssaviruses of the same genotype. Two-thirds of human patients infected with dog RABV variants show clinical signs of classic furious rabies, the remaining third develop paralytic rabies.17,18 Clinical presentation may vary from classical (furious) form of rabies and paralytic form of the disease depending on characteristics of virus isolated from the infected animal.19 However, clinical symptomatology is complex and can confuse physicians17 and atypical signs with varied symptoms have been associated with infection with either bat or dog RABV variants.9
It must be clear that RABV spillover to other species, can lead to new hosts and emergence of new viral host relationships and even new biotypes.20 Any study regarding information about any reservoir is fundamental for the understanding of the intrinsic relationship between virus and host, and then the impact in a specific population.
Considering the different pattern of pathogenicity of RABV strains the aim of this study was to provide more information about clinical evolution, level of virus replication in brain tissue through real-time polymerase chain reaction (RT-qPCR) and pattern of positivity in the fluorescent antibody test (FAT) of five strains of rabies virus (RABV) isolated from the main Brazilian reservoirs in experimentally infected mice.
Materials and methodsAnimals were housed and handled with ethical principles in animal research adopted by Bioethics Commission of the Faculty of Veterinary Medicine and Animal Production of São Paulo State University (protocol number 38/2012).
Animals and virusesFive groups of 20 Swiss mice were used totaling 100 six-week old, female, specific pathogen free, who were divided into two groups: one for clinical evaluation and daily weight control, and the remaining group for sample collection.
The intracerebral inoculation with 50 LD50/30μL suspension of rabies virus isolates was performed with RABV samples isolated from insectivorous bat (Myotis spp.), bovine (variant 3 – Desmodus rotundus), dog (variant 2 – Canis familiaris), crab eating fox (Cerdocyon thous) and marmoset (Challithrix jaccus).
Samples were antigenically characterized using the monoclonal antibody panel from the Centers for Disease Control and Prevention (CDC) in Instituto Pasteur from São Paulo. Samples from Myotis spp., crab eating fox, and marmoset were not compatible with any of the identified variants suggesting a specific viral variant in those species. The sample isolated from dogs was characterized as variant 2 and the sample collected from a rabid bovine turned out to be variant 3, which has as reservoir the hematophagous bat Desmodus rotundus.
All the 10 samples collected were submitted to FAT and qRT-PCT evaluation for N gene. Brain tissue was collected in the beginning of the agonic phase of the disease, being all samples collected ante-mortem. All brain samples were obtained at the fourth intracerebral passage in order to standardize the samples.
Mice were kept in ventilated cabinets with HEPA (High Efficiency Particulate Air) filters and feed with irradiated food and sterile water “ad libitum”. They were observed twice a day for clinical signs and weighing.
Samples were collected using sterile tweezers and scissors to cut the skin of the head and to open skullcap, respectively. The collected brain was divided into two pieces and one part stored in glycerin 50% and other part immediately placed on ice then stored at −80°C until processing. The experimental part involving animals followed the recommendation of the guide DBCA.21
Fluorescent antibody test (FAT)The presence of the RABV was investigated in brain samples by FAT and qRT-PCR for quantification of rabies virus N gene.
FAT test was performed using smears of brain sampled on clean glass microscope Multiwell Teflon® coated slides (Perfecta®). Duplicate sets of slides were prepared and stored frozen for potential repeat tests. After drying the slides were fixed overnight in acetone at −20°C then removed and thoroughly air dried. Then polyclonal anti-rabies conjugated stained with fluorescein isothiocyanate (produced by Centro de Controle de Zoonoses de São Paulo – CCZ) was applied drop-wise to each slide, which was then incubated in a moist chamber at 37°C during 30min. The slides were washed in buffered saline (three times for five minutes each), air dried then coverslips were mounted with buffered glycerol, sealed and immediately read in a light fluorescence microscope. In order to quantify positivity intensity a scale of crosses (one through three) were created using as reference the FAT of Challenge Virus Standard (CVS) strain inoculated mice slide, being classified as weak (+) when presenting 30% of the reference fluorescence, (++) when presenting 60% and (+++) when presenting 100%.
Real time RT-PCR (qRT-PCR)Viral RNA was extracted from central nervous system (CNS) fragment using a commercial kit RNeasy Lipid Mini Kit (Qiagen®) following manufacturer's protocol. The purified genomic RNA was quantified (NanoDrop®, Thermo Scientific®) in order to check RNA quality and equalize the concentration up to 1μg/μL with ultrapure DNAse/RNAse free water (IDT®) in a total volume of 6μL.
The reverse transcription was performed using Super Script II (Invitrogen®) enzyme and oligo DT as primer following the protocol: 1μL oligo DT (Prodimol®) at 10pmol, 1μL dNTP (Invitrogen®) at 10mM of concentration. Two microliters of oligo DT was added to each sample and the final volume at this step was of 8μL; the sample was homogenized and incubated at 65°C for eight minutes. After heating samples were immediately chilled on ice. Twelve microliters of a mixture containing 4μL RT-Buffer 5×, 2μL DTT (0.1mM), 4μL de MgCl2 (50mM), 1μL RNAse Out (Invitrogen®) and 1μL SuperScript II (200U/μL) were added to each sample with final volume of 20μL. The samples were incubated in a conventional PCR thermocycler following the cycle of 25°C/10min, 42°C/1h and 35min, 70°C/15min.
The qRT-PCR was then performed using 2μL of complementary DNA, 1μL of each primer (0.2μg) and 21μL of Sybr Green (Promega®), totaling a final volume of 25μL in plates with 96 wells (Applied Biosystems®). Primer P510 [sense (ATAGAGCAGATTTTCGAGACAGC)] and primer P784 [anti-sense (CCTCAAAGTTCTTGTGGAAGA)] targeting the N gene were previously described.22 All samples were processed twice in order to ensure reliability of results. Plates were incubated in Real Time PCR System ABI7500 (Fast Applied Biosystems®) following standard cycle: 40 cycles of 50°C/20s, 95°C/10min, 95°C/15s, 60°C/1min.
The positive control used for all reactions was the CVS strain and sterile DNAse/RNAse free water was used as negative controls. Positive controls were added at each batch.
GraphPad® Prism 6.0 software was used for statistical analysis. Survival rates were compared using the Mantel Cox method; incubation period, evolution period, and N gene were analyzed using Mann–Whitney test. p<0.05 was considered statistically significant.
ResultsClinical evaluationAs five RABV from different origin were studied, clinical signs varied widely. Animals inoculated with RABV variant 2 and isolated from Myotis spp. had the furious form of disease, presenting hiperexcitability, aggressiveness, ataxia, paralysis progressing to coma or agonal state and death. Mice infected with Myotis spp. sample also presented arched back and conjunctivitis. On the other hand, mice infected with variant 3, marmoset and crab eating fox virus samples developed the paralytic form. Animals were quiet, prostrated, and presented paralysis progressing to agonic state and death. All animals presenting clinical rabies eventually died. The mean incubation and evolution periods as well as the lethality rate of all the strains evaluated are presented in Table 1A.
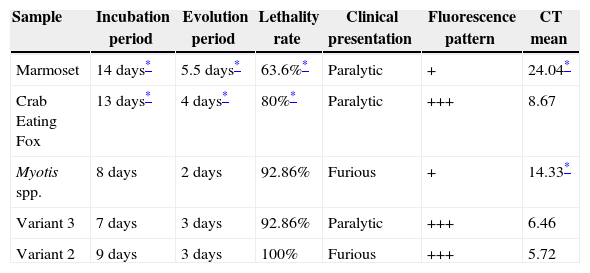
Results of incubation period, evolution period, lethality rate, clinical presentation, fluorescence pattern in FAT and CT mean in qRT-PCR in mice experimentally infected with different RABV variants.
| Sample | Incubation period | Evolution period | Lethality rate | Clinical presentation | Fluorescence pattern | CT mean |
|---|---|---|---|---|---|---|
| Marmoset | 14 days* | 5.5 days* | 63.6%* | Paralytic | + | 24.04* |
| Crab Eating Fox | 13 days* | 4 days* | 80%* | Paralytic | +++ | 8.67 |
| Myotis spp. | 8 days | 2 days | 92.86% | Furious | + | 14.33* |
| Variant 3 | 7 days | 3 days | 92.86% | Paralytic | +++ | 6.46 |
| Variant 2 | 9 days | 3 days | 100% | Furious | +++ | 5.72 |
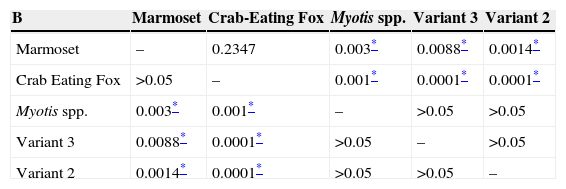
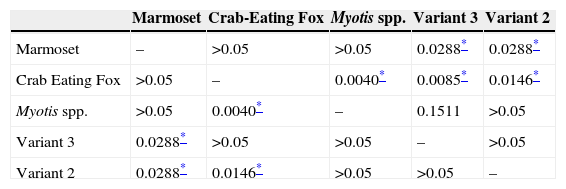
It is noteworthy that the incubation period of animals inoculated with samples isolated from crab eating fox and marmoset was longer than that observed with inoculation of variant 2, variant 3, and of Myotis spp. The longest evolution period was associated with the marmoset strain, followed by the crab eating fox strain. All the statistical results from the comparisons between groups regarding the incubation period and evolution period are presented in Tables 1B and 1C respectively. The lethality rate was significantly lower for the samples isolated from the marmoset (p<0.05) and crab eating fox (p<0.05).
Results of p values of statistical analyze of incubation period (Mann–Whitney test) made between groups.
Results of p values of statistical analyze of evolution period (Mann–Whitney test) made between groups.
The FAT turned out positive in all samples with some observed differences. Viral replication with the marmoset and Myotis spp. strains, both classified as weak (+), were lower than the other samples as fewer corpuscles were observed as well as lower fluorescence intensity. The brain from mice inoculated with virus isolated from marmoset and Myotis spp. presented discrete fluorescent corpuscles and some fluorescent dots spread throughout the tissue, while in samples related to variant 2, variant 3, and crab eating fox inclusions were larger with strong fluorescence.
Quantitative qRT-PCRViral load assessed in brain tissue was quantified by qRT-PCR with primers designed for the N gene and the Ct results are presented in Table 1A.
Samples isolated from marmoset had the highest Ct value, and thus the lowest level of replication, followed by the sample from Myotis spp. significantly different between them (p=0.0286), and both samples were significantly different when compared to variant 2, variant 3, and crab eating fox (p=0.0286).
Discussion and conclusionsRABV variants have different reservoirs in nature23 and to maintain its circulation the virus has adapt to the animal species sometimes affecting viral tropism, pathogenesis, clinical outcome of the host, establishing new host-virus relationship.20,24,25 Our results demonstrated that virus isolated from different species experimentally inoculated in mice have unique characteristics that reflect on viral replication, incubation and evolution periods, as well as in lethality rate.
Data obtained from the experimental inoculation clearly demonstrate that marmoset and crab eating fox viruses are different from the other virus strains analyzed, not only because they caused lower lethality, but also because of the longer incubation and evolution periods when compared to the other strains.
Results obtained by qRT-PCR are in agreement with FAT once lower fluorescence was observed in samples with lower rate of replication and +++ FAT had lower Ct, translating higher replication levels. However, the level of viral replication does not seem to be related to the clinical presentation once animals inoculated with Myotis spp. and variant 2 strains had similar clinical presentation but different viral load (p<0.05). In addition, samples from crab eating fox and of marmoset origin were similar regarding clinical aspects when compared to others, but presented differences regarding viral load by qRT-PCR (p<0.05). Myotis spp. and marmoset isolates had similarities in viral quantification (qRT-PCR) and pattern of positivity observed in FAT but were quite different when evolution period, lethality rate, and clinical signs were analyzed. This observation suggests that viral replication itself does not play a major role in the outcome of RABV infection in agreement with the study published by Tirawatnpong et al.26 In this study RABV antigen profile was evaluated by FAT in four encephalitic and three paralytic rabies cases, showing no correlation between viral distribution, clinical manifestations, and antigen distribution in SNC. Additionally, it demonstrated that viral replication is not related with clinical manifestations or time to evolution before and after disease.
Hemachudha et al.11 reported greater amount of rabies viral RNA in samples collected from dogs with the furious form compared with animals with paralytic presentation infected by the same virus. The authors suggested that a more extensive propagation of the virus in the CNS could be related to this form of disease. RABV isolates from different species have their own characteristics and our results demonstrate that the clinical presentation is not always related to the amount of virus in the CNS once the brain infected by Myotis spp. Strain presented low level of viral replication (high Ct and +FAT) associated with the furious form of the disease. At the same time, animals inoculated with variant 2 also presented the furious form with +++ FAT and low Ct, indicating high replication levels. Furthermore, it seems that the level of viral replication as a unique factor does not seem to be responsible for the lethality once isolates from crab eating fox had high level of replication but low rate of lethality when compared to variant 2, which presented the highest lethality rate as well as the highest replication level.
The ability of RABV to evade the immune system and reach the CNS is closely related to the intrinsic characteristics of the viral sample with several genes involved in the process. It has been demonstrated that differences in pathogenicity of recombinant strains of RABV are more related to higher level and longer duration of cytokines and chemokines expression than with viral replication.27
RABV has evolved unique characteristics to prevent viral clearance, including the mechanisms of replication that delay host cell metabolism as well as preventing neurons apoptosis, blood–brain barrier permeability alterations, which causes evasion of innate and adaptive immune response11 contributing to acceleration of death.17 The increased survival of animals inoculated with marmoset samples could be explained by the early production of cytokines, and more efficient immune response. The capacity from each sample to influence the outcome is dependent on the elicited immune response.28 The highly pathogenic rabies virus strain isolated from silver haired bats (SHRBRV) was also found to be a poor inducer of innate immune response, not inducing apoptosis in brain.29 The occurrence of a unique RABV cycle in wild animals30 is probably the result of a good host interaction, and low lethality rate is a factor that helps virus to be maintained in a specific population once high rates of lethality may not be favorable for viral transmission.31
Hoary crab eating fox virus has an independent cycle in Paraíba region in Brazil. Phylogenetic studies suggest that this isolated strain was derived from domestic dog jumping to crab eating fox.7 In our study crab eating fox isolates showed 80% lethality rate, being one of the lowest rate but with the longest evolution period. Even being related to variant 2, the crab eating fox sample here studied had biological features completely distinct, even both belonging to Canidae family. Those two strains presented significantly different incubation and evolution periods, and also in survival rates, but had no significant difference regarding N gene quantification.
Of the five viral proteins synthesized by RABV, the matrix protein is directly involved in RNA synthesis and host-cell interaction, and has reported to be associated with cytoplasmic inclusion bodies,32 which are potential sites of virus transcription and replication.10 This would explain why samples from marmoset and Myotis spp., which had low rates of replication, also showed few corpuscles by FAT. The M protein, mainly involved in viral replication, can be differentially expressed in both of these samples, but this would require further investigation.32
Although some reports show differences in clinical signs and evolution period in dogs presenting the furious and the paralytic form of the disease,11 in our study this could not be stated once mice inoculated with variant 2 only presented the furious form. Brain tissue from mice inoculated with this strain showed high levels of viral replication, both in qRT-PCR and in FAT when compared to other viral strains studied, in agreement with a report that showed a high viral load in the brain of naturally infected dogs.33
Samples from crab eating fox and marmoset presented the paralytic form of the disease with longer period of evolution and lowest lethality rate showing that a longer period of incubation may allow enough time to mount a more efficient immune response, translated in lower lethality rate in those two groups. Some studies have shown that the paralytic form is associated with prompt immune response and a high level of brainstem inflammation that would prevent the virus from entering the brain hemispheres.34 Only few cases of survival following clinical rabies, mainly related to the paralytic form of the disease, have been reported.35 Although it had been previously reported that RABV bat variants usually causes the paralytic form of the disease,36 in this study the samples from bat origin (variant 3 and Myotis spp.) caused both paralytic and furious forms.
Considering the wide range of reservoirs in nature and the complexity of individual factors and also those related to the virus itself more studies are needed to understand the complexity in rabies pathogenesis. Cunha et al.37 evaluated samples isolated from non-hematophagous bats in experimental inoculation and reported that samples isolated from Epitesicus furinalis and Molossus molossus were less pathogenic when inoculated by the intramuscular route when compared to intracerebral route demonstrating that the route of infection may also influence in the outcome of infection, regardless those already cited above, and this fact should be considered for any definitive conclusion about a RABV strain.
The progression and success or failure of the infection rely on the immune response and the success of one of the most efficient strategy that RABV has developed, namely to suppress the interferon (IFN) response from the host, which plays a major role against viral infections by acting as an antiviral cytokine, thus escaping the first line of host defense.38 The virus enters almost exclusively in neurons, where it triggers interferon and inflammatory response as any other viral infection.28 Toll like receptor 3 (TLR3) is a protein also involved in early host defense mechanism because it modulates neuronal survival.39 RABV hijacks this receptor to inside the Negri bodies using a host structure to support its replication once the Negri bodies can be considered as a location in which a massive viral replication occurs, and also avoids cellular apoptosis becoming almost invisible to the immune system.39,40
N gene quantification was performed in these samples and evaluated comparatively to FAT patterns. Considering these aspects, results showed that samples from Myotis spp. and marmoset were similar but different from crab eating fox, variant 2, and variant 3. Crab eating fox and marmoset isolates, presented different N gene quantification but similar clinical aspects, like evolution and incubation periods, and also lethality, reinforcing that clinical aspects do not seem to be related with N gene viral load detected in these samples. The difference in viral N gene expression and FAT pattern suggests that viral RNA distribution may differ among rabies variants being possibly related to the ability of hijacking TLR3 to inside the Negri bodies and thus promoting higher level of viral replication.40,41 Corpuscles were detected in different amount by FAT showing that the virus was presented in various levels in cells and virus strains being compatible with qRT-PCR results, demonstrating the ability of these techniques to assess virus load and replication, as well as the high level of agreement between both techniques.
Based on our data we can infer that replication was neither related to the clinical form of the disease (furious or paralytic) nor with the outcome (survival or death) but may be related to the immune response elicited by the virus. RABV seems to minimize the inflammation in the nervous tissues it infects and the pathogenic viral strain is characterized by inducing limited acute inflammatory response.42,43 Immune response events may be involved and influence the outcome of infection. The results demonstrate that the variants had independent characteristics that determine the clinical evolution and survival of the infected mice.
Conflicts of interestThe authors declare no conflicts of interest.
We would like to thank Luzia Fátima Alves Martorelli for the RABV conjugate.
This study was financially supported by Fundação de Amparo ‘a Pesquisa do Estado de São Paulo (FAPESP) 2012/00895-5 and 2012/00896-1.