Antiretroviral therapy and prophylaxis during the antepartum, intrapartum and postpartum periods, cesarean delivery and avoidance of breast milk significantly reduce vertical transmission of HIV.
ObjectiveTo evaluate the effectiveness prevention of mother-to-child transmission of HIV and determine the rate of vertical transmission in a public sexually transmitted infection and HIV referral center in Salvador, Bahia, in the period immediately prior to the initiation of universal antiretroviral therapy in pregnant women.
MethodsCross-sectional study using data collected from medical records of children born to HIV infected mothers in Bahia from 2005 to 2008 who were referred to the Reference Center for Diagnosis and Research of Sexually Transmitted Diseases and HIV/AIDS for care.
ResultsOf 232 HIV-exposed infants, 19 (8.2%) had confirmed HIV infection. One hundred eighty-eight (81%) mothers received antenatal care, 120 (52%) antepartum antiretroviral therapy or prophylaxis, and 168 (72%) intrapartum zidovudine. Two hundred twenty-three (96%) infants received zidovudine. In multivariable models, the combination of intrapartum and postpartum antiretroviral prophylaxis was associated with decreased adjusted odds of mother-to-child transmission.
ConclusionsLow levels of antenatal screening and access to prevention of mother-to-child transmission were significant limitations in the cascade of prevention of mother-to-child transmission at our center in this period.
Although there has been a global decline in incident HIV infection worldwide, this has been less pronounced in Latin America.1 Within Latin America, Brazil accounts for 47% of all people living with HIV.1 Brazil has a mixed epidemic with heterosexuals, injection drug users, and men who have sex with men predominating in different areas of the country.2 In northeastern Brazil, where heterosexual transmission is the most common route of transmission, there is a substantial burden of HIV infection in infants.3
Worldwide progress in stopping new HIV infections among children has been dramatic. In 2013, 240000 children were estimated to be newly infected with HIV.1 This is 58% lower than in 2002, the year with the highest incidence, when 580000 children were newly infected. Providing access to antiretroviral drugs to pregnant women living with HIV has averted more than 900000 new HIV infections among children since 2009.1 These declines have been due in large part to improved prevention technology, including widespread antenatal screening for HIV, suppressive antiretroviral prophylaxis and therapy, use of cesarean sections, and avoidance of breast feeding.4 In particular, the use of antiretroviral drugs to prevent mother-to-child transmission (MTCT) has expanded massively since the first reports of zidovudine (ZDV) prophylaxis, and many countries have initiated programs to prevent HIV vertical transmission with the result that today MTCT rates of HIV infection are as low as 1% in developed countries and <5% in developing countries.5,6
In 1996, the Brazilian government began a national program of free access to antiretroviral therapy (ART) and comprehensive HIV prevention that included mandatory antenatal HIV testing for pregnant women. Subsequently, in a nationwide multicenter study, Succi showed that MTCT rates were around 7%, ranging from 6% in the South and Central-West to 15% in the Northern Region.7,8 These rates could reach as little as 1% if all the Ministry of Health recommendations to avoid vertical transmission of HIV were fully adopted.9
We present in this study an evaluation of PMTCT at a major center in the state of Bahia in northeastern Brazil, where heterosexual transmission is the predominant form of infection in adults.
MethodsWe conducted a registry-based cross-sectional study of HIV-infected mothers and their infants to evaluate the effectiveness of the program for prevention of MTCT (PMTCT) at the Reference Center for Diagnosis and Research of Sexually Transmitted Diseases and HIV/AIDS of the State of Bahia (CEDAP). CEDAP provided care for 63.3% of HIV-infected pregnant women in Bahia during the study period. We analyzed data collected in the period of 2005–2008. This corresponds to the period in which all data were centralized in CEDAP. The standard of practice for PMTCT during the study period was defined by Brazilian national PMTCT guidelines9 and included universal antenatal screening for HIV, antiretroviral prophylaxis for both mothers and newborns, and provision of free formula to avoid exposure through breast milk.
Study populationWe included all children aged 0–36 months old born to HIV-infected mothers residing in Bahia during the study period who received their care through CEDAP. All children included in the study had at least two viral load determinations according to the surveillance definition for vertically transmitted HIV in Brazil.10
Data collectionData were collected from CEDAP's electronic medical record system. This system, called SMART, stores and manages information collected at each patient visit. We extracted data from medical, surveillance, and laboratory records, including demographic data and epidemiological data related to HIV infection and sexually transmitted infections (STI), clinical evaluations, biochemical and hematological values, HIV serology and HIV viral load.
Our primary outcome variable was HIV infection. A child was considered HIV-infected if he or she had two detectable positive viral load tests at different times. A child status was considered negative if he or she presented two or more undetectable viral loads at 6 weeks of age or later.
Data analysisWe constructed a data bank and analyzed our data using STATA version 11 (STATA Corporation, College Station, TX, USA). Our principal outcome variable was confirmed HIV infection in infants. We examined associations with confirmed infection by calculating odds ratios and testing for significance using the χ2 test. In order to select the best model we used the Akaike information criterion (AIC) for goodness of fit.11 We examined the independent contribution of predictor variables by constructing a multivariate logistic regression model.
Ethical considerationsThis project was approved by the Research Ethics Committee of the Bahia Ministry of Health.
ResultsOf the 622 children who were born to HIV-infected mothers at CEDAP between 2005 and 2008, 172 (28%) did not meet our eligibility criteria because these children were seen one or fewer times in the health service, leaving 450 eligible infants. Of these, 232 (52%) children had two or more viral loads and were available for analysis. One hundred eighty-eight (81%) women received antenatal care, 120 (52%) received antiretroviral prophylaxis during the antepartum period, and 168 (72%) received intravenous zidovudine (ZDV) during the intrapartum period. Two hundred twenty-three (96%) infants received postexposure ZDV prophylaxis. Ten (4.3%) mothers reported breastfeeding.
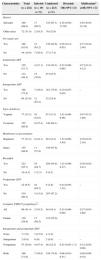
Nineteen (8.2%) of the 232 infants were infected (Table 1). Receiving antiretroviral prophylaxis in the antepartum and intrapartum periods was significantly associated with lower risk of transmission. The greatest reduction in transmission was among mother-infant pairs in which the mother received intravenous ZDV during labor and delivery and the infants received post-exposure oral ZDV (odds ratio [OR] 0.06, 95% confidence interval [CI] 0.01–0.31) (Table 1). Delivery by cesarean section was not significantly associated with transmission (OR 2.42, 95% confidence interval 0.96–6.08).
Factors associated with mother-to-child HIV transmission, Bahia, Brazil, 2005–2008.
| Characteristics | Total | Infected | Uninfected | Bivariate | Multivariate* |
|---|---|---|---|---|---|
| (n=232) | (n=19) | (n=213) | OR (95% CI) | aOR (95% CI) | |
| n (%) | n (%) | ||||
| District | |||||
| Salvador | 160 (69.0) | 17 (89.5) | 143 (67.1) | 4.16 (0.94–37.95) | 4.63 (0.93–23.10) |
| Other areas | 72 (31.0) | 2 (10.5) | 70 (32.9) | ||
| Pre-natal care | |||||
| Yes | 188 (81.0) | 12 (63.2) | 176 (82.6) | 0.36 (0.14–0.95) | 0.77 (0.24–2.46) |
| No | 44 (19.0) | 7 (36.8) | 37 (17.4) | ||
| Antepartum ART | |||||
| Yes | 120 (51.7) | 4 (21.1) | 116 (54.5) | 0.22 (0.08–0.66) | 0.73 (0.13–4.12) |
| No | 112 (48.3) | 15 (78.9) | 97 (45.5) | ||
| Intrapartum ART | |||||
| Yes | 168 (72.4) | 7 (36.8) | 161 (75.6) | 0.19 (0.07–0.49) | – |
| No | 64 (27.6) | 12 (63.2) | 52 (24.4) | ||
| Type of delivery | |||||
| Vaginal | 77 (33.2) | 10 (52.6) | 67 (31.5) | 2.42 (0.96–6.08) | 0.47 (0.12–1.80) |
| Cesarean | 155 (66.8) | 9 (47.4) | 146 (68.1) | ||
| Membranes at presentation | |||||
| Ruptured | 77 (33.2) | 8 (42.1) | 69 (32.4) | 1.52 (0.60–3.84) | 0.70 (0.23–2.16) |
| Intact | 155 (66.8) | 11 (57.9) | 144 (67.6) | ||
| Breastfed | |||||
| Yes | 222 (95.7) | 18 (94.7) | 204 (95.8) | 1.25 (0.00–8.27) | 0.30 (0.02–4.41) |
| No | 10 (4.3) | 1 (5.3) | 9 (4.2) | ||
| Postpartum ZDV | |||||
| Yes | 22 (9.5) | 16 (84.2) | 6 (2.8) | 0.24 (0.05–1.22) | – |
| No | 210 (90.5) | 3 (15.8) | 207 (97.2) | ||
| Complete PMTCT prophylaxis** | |||||
| All | 96 (41.4) | 2 (10.5) | 94 (44.1) | 0.15 (0.00–0.60) | 0.27 (0.03–2.80) |
| Partial | 136 (58.6) | 17 (89.5) | 119 (55.9) | ||
| Intrapartum and postpartum ZDV | |||||
| None | 7 (3.0) | 3 (15.8) | 4 (1.9) | ||
| Intrapartum | 2 (0.9) | 0 (0) | 2 (0.9) | – | – |
| Postpartum | 57 (24.6) | 9 (47.4) | 48 (22.5) | 0.25 (0.05–1.3) | 0.12 (0.02–0.86) |
| Both | 166 (71.6) | 7 (36.8) | 159 (74.6) | 0.06 (0.01–0.31) | 0.04 (0.00–0.35) |
aOR, adjusted odds ratio; ART, antiretroviral therapy; CI, confidence interval; PMTCT, prevention of mother-to-child HIV transmission; ZDV, zidovudine.
In multivariate logistic regression, we found that the combination of intrapartum ZDV in the mother and provision of ZDV to the infant had the lowest adjusted odds of transmission (adjusted OR [aOR] 0.04, 95% CI 0.00–0.35). Prophylaxis in newborns alone also independently predicted lack of transmission (aOR 0.12, 95% CI 0.02–0.86).
DiscussionWe found that the rate of MTCT in the principal public-sector provider in Bahia in the period 2005–2008 was 8.2%, which is substantially higher than rates that have been achieved in other parts of Brazil.12 This period was characterized by suboptimal coverage of antenatal HIV screening and antepartum prophylaxis, suggesting that as these components of the PMTCT achieve greater coverage, transmission rates should fall as well. More recent national-level data on screening and prophylaxis in Brazil have associated improvements in the national PMTCT program with significant declines in transmission rates.9 We were also able to confirm that the combination of intrapartum and postpartum ZDV prophylaxis was associated with decreased adjusted odds of transmission in this real-world setting, as was infant post-partum prophylaxis alone.
The impact of the PMTCT on the prevalence of vertical transmission of HIV infection varies significantly among the different regions of Brazil.3,13 In São Paulo the rates of perinatal transmission of HIV infection among babies born to HIV-infected mothers fell from 16% in 1995 to 2.7% in 2006.6 In Goiás, a large state in the central region, 70 (21%) of 332 HIV-exposed children were infected in the period 1995 to 2001.14 In a multicenter study conducted in Brazil in 2000, including states from the five largest regions of the country, rates of vertical transmission varied between 4.9% to 17.6% with the highest rate in the North of the country. Interestingly, HIV MTCT rates among women with and without antenatal care were 6.5% and 28.7%, respectively, and fell to between 5.6% and 18.0% in 2001.7 Sentinel surveillance in Brazil in 2002 clearly indicated regional inconsistencies in offering HIV testing to pregnant women during antenatal care or even at delivery, varying from 24% in the Northeast to 72% in the South. Overall, the coverage of antenatal HIV testing in this study was only 52%.15 An earlier Brazilian study of the effectiveness of intrapartum and postpartum ZDV prophylaxis found results similar to ours, with risk of MTCT in Campinas decreasing from 32.2% to 2.9% after the PACTG 076 protocol was implemented.16 The MTCT rate in the State of Pernambuco was 9.2%17 very similar to a rate of 9.9% recorded in Manaus, in the northern region, between 2007 and 2009.18 One study in Rio Grande do Sul between 2003 and 2007 found a transmission rate of 4.8%, and the decline over the years was attributed to the successful control of factors, which causes peripartum and postnatal transmission.19
Our study has significant limitations. First, because we required two negative viral tests for a child to be defined as uninfected, we had substantial missing data and were only able to study a sub-sample of all HIV-exposed infants reported through CEDAP during this time period. If all exposed pregnancies had been detected, the transmission rate we observed may well have been different from what would have been found if complete data were available both because of increased power as well as the potential for non-random missingness. Secondly, the study was dependent on medical records and surveillance data, with problems of data quality and completeness. Finally, while CEDAP provided care for approximately two-thirds of all HIV-infected pregnant women in Bahia, our sample is not representative of the entire state. Inclusion of patient from other referral centers and from more rural areas may well have led to different results.
Nonetheless, within the constraints of the data available to us, we believe that we have a reasonable estimate of coverage of critical PMTCT interventions in the major referral center in Bahia during this period. Additional effort needs to be made to improve data collection system to assure a more complete sample of the universe of exposed infants born in Bahia. Assuring universal antenatal screening and access to PMTCT before pregnant HIV-infected women present in labor have improved in Bahia, especially as the Brazilian government implements its new strategy of universal life-long ART at diagnosis, including for pregnant women.20
Authors’ contributionFRLP designed the study, collected and analyzed the data and wrote the manuscript. GWR assisted in data analysis and edited the manuscript. JHSB assisted in study design and data collection. CR assisted in study design and data collection. VB assisted in data collection and analysis and in writing. RB assisted in study design, oversaw data collection and analysis and edited the manuscript. All authors approved the manuscript before submission.
FundingThis work was supported by the Health Secretary of State, Government of Bahia, and by a grant from the National Institutes of Health, Fogarty International Center through the ICOHRTA Brazilian Scientists Program (D43 TW05799).
Conflicts of interestThe authors declare no conflicts of interest.
We gratefully acknowledge the support of CEDAP staff for their work in gathering these data. We thank Drs. Vilmar Bião, Mauricio Cardeal and Carlos Teles for their kind assistance with our statistical analyses.