This study aimed to determine the excess length of stay, extra expenditures, and attributable mortality to healthcare-associated S. aureus bloodstream infection (BSI) at a teaching hospital in central Brazil. The study design was a matched (1:1) case-control. Cases were defined as patients >13 years old, with a healthcare-associated S. aureus BSI. Controls included patients without an S. aureus BSI, who were matched to cases by gender, age (± 7 years), morbidity, and underlying disease. Data were collected from medical records and from the Brazilian National Hospital Information System (Sistema de Informações Hospitalares do Sistema Único de Saúde – SIH/SUS). A Wilcoxon rank sum test was performed to compare length of stay and costs between cases and controls. Differences in mortality between cases and controls were compared using McNemar's tests. The Mantel-Haenzel stratified analysis was performed to compare invasive device utilization. Data analyses were conducted using Epi Info 6.0 and Statistical Package for Social Sciences (SPSS 13.0). 84 case-control pairs matched by gender, age, admission period, morbidity, and underlying disease were analyzed. The mean lengths of hospital stay were 48.3 and 16.2 days for cases and controls, respectively (p<0.01), yielding an excess hospital stay among cases of 32.1 days. The excess mortality among cases compared to controls that was attributable to S. aureus bloodstream infection was 45.2%. Cases had a higher risk of dying compared to controls (OR 7.3, 95% CI 3.1-21.1). Overall costs of hospitalization (SIH/SUS) reached US$ 123,065 for cases versus US$ 40,247 for controls (p<0.01). The cost of antimicrobial therapy was 6.7 fold higher for cases compared to controls. Healthcare-associated S. aureus BSI was associated with statistically significant increases in length of hospitalization, attributable mortality, and economic burden. Implementation of measures to minimize the risk of healthcare-associated bacterial infections is essential.
Bloodstream infections (BSIs) are serious and potentially fatal events associated with high morbidity and mortality rates. Mortality rates among patients with BSIs vary across studies, most likely due to patient co-morbidities and etiological agents.1–4 Although BSIs are not the most frequent healthcare-associated infection,5 they have been associated with heavy clinical and economic burdens in many settings.6–19 In general, mortality rates associated with BSIs are high worldwide, in spite of technological advances and availability of effective antimicrobial drugs.1,3,16,19–21 Although a consensus on the topic has not been reached, several studies have shown a significantly higher attributable mortality due to healthcare-associated BSIs.3,6,8,9,13,16 In contrast, other studies did not find differences in mortality rates between patients with and without BSIs, after controlling for some potential confounders such as age and comorbidities.12,22 Discrepant results across studies may reflect differences in study methodology, severity of infection, hospital conditions, or bacteriological profiles.
Although the bacteriological profile of healthcare-associated BSIs varies with time period, country, and hospital, S. aureus is one of the most frequently isolated BSI pathogens worldwide.4,21,23,24S. aureus BSIs have been associated with unfavorable clinical outcomes in many studies.3,25 Some studies showed higher impact on costs and mortality rates due to methicillin-resistant S. aureus (MRSA), compared to methicillin-susceptible strains (MSSA).10,26–29 Few studies have investigated the impact of S. aureus on costs and attributable mortality rates in Latin America.9,26 The objective of the current study was to estimate increased hospital stay, increased direct costs, and mortality attributed to healthcare-associated BSI caused by S. aureus, in patients admitted to a teaching hospital in Brazil.
MethodsPopulation and settingThis study was conducted in a public, tertiary-care teaching hospital in the city of Goiânia, the capital of the Brazilian state of Goiás, which has a population of approximately 1.2 million. During the study period (2000-2001), approximately 12,000 patients per year had been admitted to that hospital; 118 patients had a microbiologically confirmed BSI due to S. aureus. Blood cultures were taken on a routine basis when infection was suspected. A total of 34 cases were excluded from the analysis: seven had incomplete clinical data to rule out contamination, 24 cases had community acquired BSI, and three cases were younger than 13 years old. The remaining 84 adults had at least one episode of clinically significant healthcare-associated BSI. All clinical and laboratory data were reviewed by an infectious diseases specialist physician. More detailed information has been published previously.25
Study designThis was a 1:1 matched case-control study, conducted from 2000 to 2001. Patients older than 13 years of age, with an episode of clinically significant healthcare-associated BSI caused by S. aureus were classified as cases. Controls were selected from patients without a positive blood culture hospitalized during the same period as the cases. Controls were matched by gender, age (±7 years), morbidity, and underlying disease according to the 10th edition of the International Classification of Diseases and Related Health Problems (ICD-10). In addition, the length of hospital stay of the controls had to be as close as possible to the length of stay of the cases, from admission until the onset of BSI.
Data collection and definitionsClinical and microbiological data were abstracted from medical records and hospital laboratory reports. Financial data were extracted from the Brazilian National Hospital Information System (Sistema de Informações Hospitalares do Sistema Único de Saúde – SIH/SUS). Infections were classified as healthcare-associated when they occurred at least 48hours after hospital admission and were not complications of infections present upon admission, or when infections were detected following hospital discharge but were related to the previous admission.30 Healthcare-associated BSIs were confirmed by a S. aureus positive blood culture (BACTEC 9120-Becton Dickinson) in the presence of at least one of the following clinical manifestations: fever or hypothermia defined by an axillary temperature >38°C or<36°C, respectively; cardiac rate higher than 90 bpm; respiratory rate higher than 20 ipm or PaCO2<32mmHg; leukocyte count >12,000 leucocytes/mm3 or<4,000 leucocytes/mm3, or >10% of immature forms; organ disorder, hypoperfusion, or hypotension; or persistent sepsis-induced hypotension in spite of adequate fluid replacement.30,31
The severity of the underlying disease at admission was evaluated according to the criteria proposed by McCabe and Jackson, with modifications, and was classified into three groups: rapidly fatal disease, potentially fatal disease, and non-fatal disease or absence of underlying disease.32
Attributable mortality was defined as the excess mortality caused by BSI and was calculated by subtracting the mortality rate of the control patients from the mortality rate of the case patients with BSI. The excess length of hospital stay and the excess duration of use of invasive devices were each defined as the difference between the values obtained for cases and controls. The excess cost attributed to healthcare-associated BSIs was defined as the difference in overall costs between cases and controls. Cost data were obtained from the Brazilian National Public Health Reimbursement System for Hospital Admission (Sistema de Informação Hospitalar Data – SUS).33 Reimbursements are made using predefined values for each particular disease or health state. This compensation system does not account for costs due to antimicrobials, parenteral nutrition, nor blood products used during the hospitalization. The medical records of each patient were reviewed to check for antimicrobials, blood products, and parenteral nutrition required for cases and controls. Estimates of costs for antimicrobial agents were based on the reference table used by private health insurance plans. Costs were calculated in Brazilian reais (R$) and then converted to United States dollars (US$) using the exchange rate of 2001.
Statistical analysisComparisons between cases and controls were conducted using Student's t-test for continuous variables and the Fisher's exact test or chi-squared tests for categorical variables. The Wilcoxon rank-sum test was used to compare length of hospital stay and direct hospital costs between cases and controls. McNemar's test was used to compare mortality rates between cases and controls. A Mantel-Haenszel stratified analysis, according to ICU admission, was performed to evaluate invasive device utilization, of both cases and controls. Odds ratios (ORMH) and 95% confidence intervals (95% CIs) were calculated.
p-values of less than 0.05 were considered to be statistically significant.
Data analyses were conducted using Epi Info 6.0 and Statistical Package for Social Sciences version 13.0 (SPSS). The study was approved by the Ethics Committee for Research on Humans and Animals of the Universidade Federal de Goiás.
ResultsEighty four cases of healthcare-associated S. aureus BSI in patients older than 13 years of age were identified. These cases were successfully mached, resulting in 84 case-control pairs matched by gender, age, admission period, morbidity, and underlying disease. Male patients represented 57.0% of cases. Participants’ ages ranged from 14 to 91 years; no difference between the mean ages of cases and controls was detected (p=0.73). Cases and controls did not differ with respect to the severity of the underlying disease at admission (p=0.44), the presence of co-morbidities (p=0.31), or the use of immunosuppressant therapies (p=0.10). Diagnoses of diabetes mellitus and chronic renal failure were more frequent among cases, compared to controls, and the risk of presenting at least one episode of healthcare-associated infection was 9.7 times higher among cases, compared to controls (Table 1). Among the 84 cases, 49 (58.3%) had a BSI due to MRSA. The median length of hospital stay prior to hospital-associated BSI was 18.5 days, ranging from two to 154 days. Cases were more frequently admitted to the intensive care unit (ICU) compared to controls (p<0.01). A stratified analysis for invasive device utilization, adjusted for ICU admission, disclosed that cases required central venous catheters (ORMH 73.8, 95% CI 20.4-309.3) and mechanical ventilation (ORMH 6.3, 95% CI 1.8-22.9) more frequently than controls. No statistically significant difference was found between cases and controls regarding the use of either urinary catheters (ORMH 2.2, 95% CI 0.9-5.1), or surgical procedures (ORMH 1.2, 95% CI 0.6-2.6). Cases received more parenteral nutrition (ORMH 6.6, 95% CI 2.1-74.5) than controls.
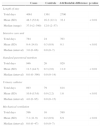
Baseline characteristics of 84 cases with healthcare-associated S. aureus bloodstream infections (BSIs) and 84 matched controls.
| Characteristics | Cases | Controls | p-value |
|---|---|---|---|
| n=84 (%) | n=84 (%) | ||
| Gender | |||
| Male | 47 (57.0) | 47 (57.0) | - |
| Age (years) | |||
| Mean (SD) | 47.7 (19.1) | 48.7 (18.1) | 0.73 |
| IQR (25% - 75%) | 33.0 – 62.5 | 35.5-63.0 | |
| Severity of underlying disease | |||
| Rapidly fatal | 6 (7.1) | 4 (4.8) | 0.44 |
| Fatal | 28 (33.4) | 26 (31.0) | |
| Nonfatal disease or absence of underlying disease | 50 (59.5) | 54 (64.2) | |
| Presence of co-morbidities | |||
| None | 22 (26.2) | 28 (33.3) | 0.31 |
| At least one | 62 (73.8) | 56 (66.6) | |
| Type of co-morbidities | |||
| Immunosuppressive therapy | 25 (29.8) | 16 (19.0) | 0.11 |
| Diabetes mellitus | 21 (25.0) | 12 (14.3) | 0.03 |
| Chronic renal failure | 21 (25.0) | 7 (8.3) | <0.01 |
| Cancer | 5 (5.9) | 5 (5.9) | 1.00 |
| Episodes of infections other than BSI | |||
| Healthcare-associated | 282 (335.7) | 29 (34.5) | <0.01 |
| Community-acquired | 18 (21.4) | 13 (15.5) | 0.32 |
SD, standard deviation; IQR, interquartile range.
The raw mortality rates for cases and controls were 57.1% (48/84) and 11.9% (10/84), respectively (p<0.01). Attributable mortality to S. aureus BSI was 45.2%. Mortality associated with BSI was higher in cases compared to controls (OR 7.33, 95% CI 3.11-21.1).
The median length of hospital stay among cases was 37 days, ranging from two to 390 days. The median length of hospital stay among controls was 12 days, ranging from two to 57 days. An excess of 2,700 hospitalization days was attributed to BSI among cases, compared to controls. A statistically significant difference was observed between cases and controls regarding the mean duration of use of invasive devices, such as urinary catheter and mechanical ventilation. Compared to controls, cases had 763 excess ICU days, in addition to a longer duration of use of invasive devices, including parenteral nutrition, mechanical ventilation, and urinary catheter (Table 2).
Length of stay and exposure to invasive procedures of 84 cases with healthcare-associated S. aureus bloodstream infections (BSIs) and 84 matched controls.
| Cases | Controls | Attributable difference | p-value | |
|---|---|---|---|---|
| Length of stay | ||||
| Total days | 4061 | 1361 | 2700 | |
| Mean (SD) | 48.3 (52.8) | 16.2 (12.1) | 32.1 | <0.01 |
| Median (range) | 37.0 (2–390) | 12.0 (2–57) | ||
| Intensive care unit | ||||
| Total days | 784 | 24 | 763 | |
| Mean (SD) | 9.4 (14.8) | 0.3 (0.9) | 9.1 | <0.01 |
| Median (interval) | 3.0 (0–66) | 0.0 (0–7) | ||
| Standard parenteral nutrition | ||||
| Total days | 946 | 26 | 920 | |
| Mean (SD) | 11.3 (44.7) | 0.3 (2.0) | 11.0 | <0.01 |
| Median (interval) | 0.0 (0–390) | 0.0 (0–14) | ||
| Urinary catheter | ||||
| Total days | 893 | 79 | 814 | |
| Mean (SD) | 10.6 (15.6) | 0.9 (2.2) | 1.6 | <0.01 |
| Median (interval) | 4.0 (0–95) | 0.0 (0–15) | ||
| Mechanical ventilation | ||||
| Total days | 596 | 16 | 580 | |
| Mean (SD) | 7.1 (11.9) | 0.2 (0.9) | 6.9 | <0.01 |
| Median (interval) | 0.0 (0–47) | 0.0 (0–7) | ||
SD, standard deviation.
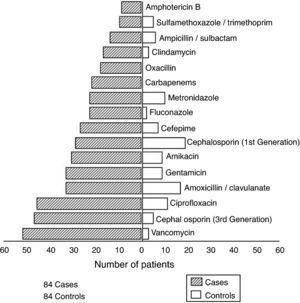
The average number of episodes of healthcare-associated BSI per case was 3.4; the average number of episodes of healthcare-associated BSI per control was 0.3. Among cases, 71 pathogens, in addition to S. aureus, were isolated from various body sites during hospitalization. Gram-negative bacilli represented the majority of isolates. Fig. 1 presents antimicrobial agents prescribed for cases and controls. Cases were prescribed more antimicrobials than controls. Fifty-two out of 84 cases received vancomycin, while only three controls required this drug.
The total hospitalization direct costs (SIH-SUS) were US$ 123,065 (US$ 1,465/patient) for cases, and US$ 40,247 (US$ 479/patient) for controls. The hospitalization cost for the cases was 3.1 times higher compared to controls. The excess hospitalization cost attributable to healthcare-associated S. aureus BSI of cases compared to controls was US$ 82,818. Costs due to use of antimicrobial agents were US$ 119,210 among cases, and US$ 17,650 among controls. The total cost attributable to BSI was US$ 101,559. The mean costs due to antimicrobial agents were US$ 1,419 per case and US$ 210 per control. Antimicrobials expenditure for cases was 6.7 times higher than for controls (Table 3).
Comparison of direct costs (US$) for 84 patients with S. aureus hospital acquired bacteraemia and their matched controls.
| Costs (US$) | All patients | |||||
|---|---|---|---|---|---|---|
| Cases (n=84) | Controls (n=84) | Difference attributed to infection | Reason(Cases/controls) | |||
| Total | For individual | Total | For individual | |||
| Total cost AH | 123065 | 1465 | 40247 | 479 | 82818 | 3.1 |
| Antimicrobials | 119210 | 1419 | 17651 | 210 | 101559 | 6.7 |
| Daily ICU | 21580 | 257 | 1058 | 13 | 20522 | 20.4 |
| Standard parenteral nutrition | 10655 | 127 | 293 | 3 | 10362 | 36.4 |
| Blood products | 9950 | 118 | 1135 | 13 | 8815 | 8.8 |
US$, dolar; AH, authorization for hospitalization; ICU, intensive care unit.
An analysis of direct costs among the subset of patients who survived BSI, including 36 cases and 74 controls, showed that costs were US$ 38,647 (US$ 1,073/patient) among cases and US$ 34,230 (US$ 462/patient) among controls. Considering expenses among survivors, the reimbursement cost for cases was 1.1 times higher than for controls. The ICU cost among cases was 20.4 times higher than among controls.
DiscussionThis study identified an increased length of hospital stay, a higher mortality rate, and an excess of direct costs related to healthcare-associated S. aureus BSI in a teaching hospital in central Brazil. The difference in mortality rates between cases and controls was 45.2%, suggesting that approximately half of the deaths could be attributed to BSI. Therefore, mortality could be reduced, at least in part, by adopting effective measures to control hospital infections. The cases enrolled in the study were more frequently admitted to the ICU, and experienced a longer duration of use of invasive procedures, compared to controls which could have been due to cases being generally more ill. However, cases and controls were not different at admission regarding reasons for hospitalization or severity of underlying disease. Cases and controls were also similar with respect to the presence of co-morbidities, reinforcing the hypothesis that differences in morbidity and mortality rates could be attributed to healthcare-associated BSIs.
The attributable mortality to healthcare-associated BSI reported herein has been described in a few previously published studies conducted in Latin America.9,26 Studies conducted around the world, mainly in the United States and Europe, have shown that hospital-acquired BSIs were associated with significant attributable mortality.3,7,11,15,17 In contrast, other studies have not demonstrated differences in attributable mortality due to hospital-acquired BSIs in ICU patients, although increased hospital costs were evident.12,18
Methodological issues, such as the selection of matching variables, heterogeneous patient populations, and miscellaneous microbiological agents causing BSIs could partially explain these divergent results. Another factor that might have influenced results, specifically with respect to total costs of treating BSIs, was susceptibility to antimicrobial agents. Other investigators demonstrated higher costs when treating MRSA BSIs vs. MSSA BSIs.27
Discussions of methodological issues in epidemiologic studies of outcomes related to healthcare-associated BSIs include the best strategies to evaluate attributable mortality, excess costs, and increased duration of hospital stays.3,8,34 Comparison of infected patients (cases) and non-infected patients (controls) matched by a given parameter would partially reduce the bias created by the severity of the underlying disease. Another approach aimed at increasing the homogeneity of the study population is to define one etiological agent (S. aureus) and one infection site.6,12 Yet another strategy would be the use of severity scores that would serve to compare cases and controls. However, severity scores or scales, such as the McCabe and Jackson score, Acute Physiology and Chronic Health Evaluations (APACHE), and the Simplified Acute Physiology Score (SAP) were developed to evaluate the risk of death during hospitalization, and they have not been validated for other purposes.
In this study, cases remained in the hospital an average of 32.1 days longer than controls. The cases also stayed longer in the ICU than controls and also required a larger number of invasive procedures, possibly attributable to the BSI. An association between BSI and increased length of hospital stay has been described previously; estimates of excess length of hospital stay have ranged widely from 1.2 to 32 days.6–9,17,19,35,36 Differences across studies may be partially explained by limiting the study samples to survivors or by choices of matching variables.
Patients hospitalized longer are at greater risk of contracting a healthcare-associated infection; however, length of hospital stay is also a marker for the severity of the underlying disease. Therefore, an important matching variable would be the length of hospital stay previous to BSI. The length of hospital stay among controls should be greater than or equal to the length of hospital stay prior to BSI for cases. Approximately 40% of cases in the present study had a hospitalization length prior to bacteremia that were longer than that of the matched control. This may suggest that cases could had more severe diseases, and points to a possible overestimation of both length of hospital stay and costs among cases, compared to controls.
It should also be noted that the sum of the direct costs for antimicrobial agents among cases represented 96.9% of the total amount paid to the hospital as reimbursement according to SIH-SUS. For the controls, direct costs for antimicrobial agents represented 45.7% of the total amount paid to the hospital as reimbursement.
In the present study, the percentage of MRSA among cases with healthcare-associated BSI was high, similar to comparable data from studies conducted in several centers in the Americas and Europe.13,23,25,26 In addition to economic implications, the large-scale use of vancomycin may contribute to an increased prevalence of resistant microorganisms.37
This study showed that healthcare-associated S. aureus BSIs are associated with higher mortality, longer hospital stays, and increased costs. This knowledge reinforces the institutional responsibility to develop strategies for effective infection prevention.
Conflict of interestAll authors declare to have no conflict of interest.
The authors would like to thank National Council for Scientific and Technological Development (CNPq) for the research scholarships granted to Marília D. Turchi (Proc. no. 306928/2010-8) and Celina M.T. Martelli.