The factors associated with bacterial vaginosis in women with homosexual, bisexual and heterosexual practices are still poorly explored. Thus, the aim of this study was to analyze the factors associated with bacterial vaginosis in women with different sexual practices.
MethodsCross-sectional study that included 453 women, 149 Women with Homosexual practice (WSW); 80 bisexual Women (WSWM) and 224 Women with heterosexual practice (WSM). The diagnosis of bacterial vaginosis was performed by microscopic examination of the vaginal smears stained by Gram method and classified according to the Nugent et al. (1991) score. Data analysis was performed by Cox multiple regression.
ResultsBacterial vaginosis was associated to years of education among WSW (0.91 [95% CI 0.82‒0.99]; p = 0.048) and non-white skin color (2.34 [95% CI 1.05‒5.19]; p = 0.037) between WSWM. Changing partners in the last 3-months (2.09 [95% CI 1.14‒3.82]; p = 0.017), inconsistent use of condoms (2.61 [95% CI 1.10‒6.20]; p = 0.030) and positive diagnosis of Chlamydia trachomatis (2.40 [95% CI 1.01‒5.73]; p = 0.048) were associated with bacterial vaginoses only in WSH.
ConclusionsThe factors associated to bacterial vaginosis differ between different sexual practices, suggesting that the type of sexual partner may influence the risk of developing this classic dysbiosis.
Bacterial Vaginosis (BV) is a common vaginal dysbiosis among women of reproductive age1,2 and it is characterized by an overgrowth of multiple anaerobes species and diminished or absence vaginal Lactobacillus spp. dominance. BV is associated with an increased risk of adverse urogenital and reproductive health outcomes, including higher risk to acquire Sexually Transmitted Infections (STI) and HIV.2-6
Among the factors associated with BV there are those associated with sexual activity, such as the number of partners,1,7 receive vaginal sex,1 and use of sexual accessories,1,8 as well as factors related to clinical variables, like use of contraceptives and the period of the menstrual cycle.1,9 In addition, there is also the sociodemographic factors, which can be related to African-American ethnicity, educational level, and age.1
Studies that included in their samples both women with a history of sexual partnership exclusively with women and with women and men found as factors associated with BV: etinicithy,10 number of female sexual partners,10-13 smoking,10,11 receiving oral-anal sex,13 use of sexual accessories,8 sharing sexual accessories without hygiene,13 and a positive BV diagnosis in sexual partners.12
However, there is a lack of studies in the literature that analyzed factors associated with BV comparing groups of women with different types of sexual partners. These findings may help to elucidate gaps in knowledge about the relationship between sexual behavior and BV. Thus, the aim of this study was to analyze the factors associated with BV in women with homosexual, bisexual and heterosexual sexual practices.
Material and methodsCross-sectional study, part of a broader study that evaluated the vulnerability of women who have sex with women to BV, conducted in a medium-sized municipal, located in the middle of the state of São Paulo, Brazil.
The sample consisted of 453 women divided into three groups: WSW group composed of 149 women with homosexual practice; WSWM group composed of 80 women with bisexual practice and WSM group that included 224 women with heterosexual practice. The classification was based on reported history of sexual partners in the last 12 months. The inclusion criteria were women with 18 years old or older, non-menopausal and have active sexual life. Women who did not accept to participate in all stages of the study (answering the questionnaire and performing the gynecological examination), those who declared themselves transgender men or transgender women submitted to gender-affirming surgery and those who had inadequate laboratory samples were excluded from the study.
The samples were constituted at two different times: from January 2015 to April 2017 and from January 2019 to January 2020. They were conducted simultaneously with two broader research projects that investigated sexual and reproductive health of women who have sex with women.
Data were obtained by interview and gynecological exams conducted by trained nurses. The questionnaires were from the previous and broader research, contemplating the variables of interest, submitted to the evaluation of specialists in the area and previously tested.
The gynecological exam was performed respecting the following criteria: sexual abstinence and no vaginal procedures in the last 72 h, last menstrual period at least five days and absence of use of antibiotics in the last 30 days prior the exam. For the vaginal inspection, and for obtaining the samples from cervix and the middle third of the vaginal and endocervical wall a Collins bivalve speculum, free of lubricant, was used.
The diagnosis of BV was performed by microscopic examination of the vaginal smears stained by the Gram method and classified according to Nugent14 scoring criteria. For the detection of Human Papillomavirus (HPV) infection in endocervical samples two kits were used. The Ampli Lute Liquid Media Extraction DNA Extraction Kit (Roche Molecular Systems, Inc.) for samples collected between January from 2015 to April 2017, and XGEN MULTI HPV CHIP Kit for samples collected from January 2019 to January 2020. The evaluation of Chlamydia trachomatis was performed with specific primers for Polymerase Chain Reaction (PCR): CT1 (5′ TAG TAA CTG CCA CTT CAT CA 3′), CT2 (5′ TTC CCC TTG TAA TTC GTT GC 3′), PL6.1 (5′ AGA GTA CAT CGG TCA ACG A 3′) and PL6.2 (5′ TCA CAG CGG TTG CTC GAA GCA 3′). PCR was performed using Go Taq Green master mix (Promega Corporation, USA) as previously described.15 Amplification parameters consisted of 40 cycles of 60 s at 95 °C, 60 s at 55 °C and 90 s at 72 °C. The human β-globin target was co-amplified to determine sample adequacy.
The independent variables analyzed were ethnicity, years of education, living with a partner, remunerated activity, per capita family income, use of tobacco, changing sexual partners in the last three months, sexual accessories, vaginal penetration, anal penetration, oral sex, inconsistent use of condom, vaginal douche, HPV, C. trachomatis, attend primary health care units and gynecological appointment in the last year. The outcome studied was positive BV diagnosis.
Descriptive data to identify difference in characteristics between WSW, WSWM and WSM were analyzed using the Chi-Square or Fisher's exact test, and Mann-Whitney. To verify the factors associated with BV in each groups studied, Cox multiple regression models were adjusted. The variables that reached p < 0.20 in the bivariate analyzes were introduced in the final models. Associations were considered statistically significant when p < 0.05. All statistical analyzes were performed using SPSS 22.0 software.
The study was approved by the local Research Ethics Committee, and it complies with all standards for research involving human beings (CAAE: 98,934,918.3.0000.5411). All participants in this study were clarified about the aims and their participation and, those who accepted, signed a term of written consent. All women diagnosed with alterations in the vaginal microbiota and lower genital tract infections were guided and treated according to the protocol of the Ministry of Health of Brazil, as well as their partners when indicated.
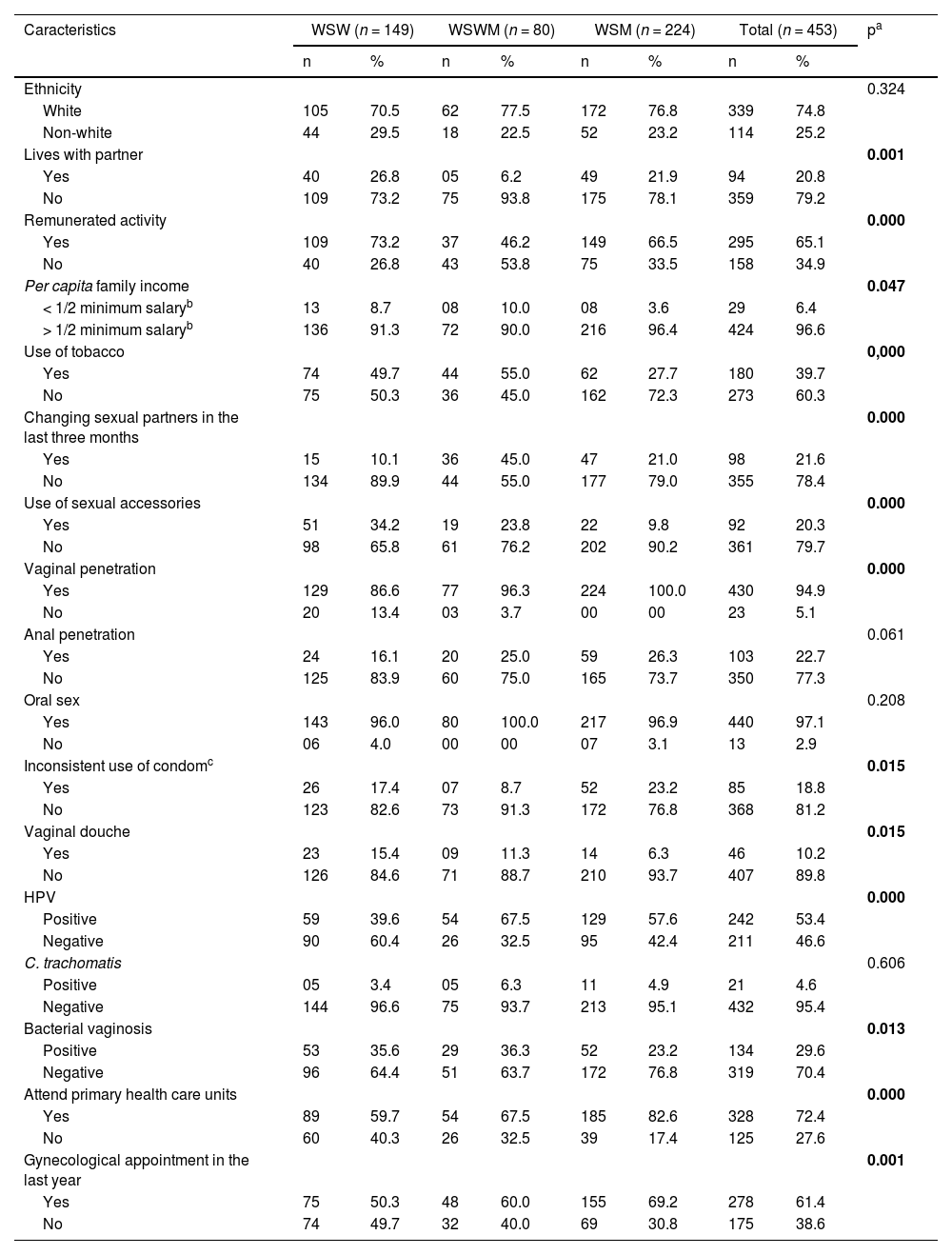
ResultsThe medians for age, years of education and per capita family income of the 453 women included in this study were: 26 (18‒55), 14 (5‒25) and R$ 1500.00 (R$ 133.00 – BRL 17,500.00), respectively.
Most women self-reported as white (74.8%), did not live with a partner (79.2%), had remunerated activity (65.1%), per capita family income > 1/2 minimum salary (96.6%), received vaginal penetration (94.9%) and oral sex (97.1%), did not use condoms consistently (81.2%) and had HPV infection (53.4%) (Table 1).
Demographic, behavioral and clinical characteristics of the studied groups.
| Caracteristics | WSW (n = 149) | WSWM (n = 80) | WSM (n = 224) | Total (n = 453) | pa | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| Ethnicity | 0.324 | ||||||||
| White | 105 | 70.5 | 62 | 77.5 | 172 | 76.8 | 339 | 74.8 | |
| Non-white | 44 | 29.5 | 18 | 22.5 | 52 | 23.2 | 114 | 25.2 | |
| Lives with partner | 0.001 | ||||||||
| Yes | 40 | 26.8 | 05 | 6.2 | 49 | 21.9 | 94 | 20.8 | |
| No | 109 | 73.2 | 75 | 93.8 | 175 | 78.1 | 359 | 79.2 | |
| Remunerated activity | 0.000 | ||||||||
| Yes | 109 | 73.2 | 37 | 46.2 | 149 | 66.5 | 295 | 65.1 | |
| No | 40 | 26.8 | 43 | 53.8 | 75 | 33.5 | 158 | 34.9 | |
| Per capita family income | 0.047 | ||||||||
| < 1/2 minimum salaryb | 13 | 8.7 | 08 | 10.0 | 08 | 3.6 | 29 | 6.4 | |
| > 1/2 minimum salaryb | 136 | 91.3 | 72 | 90.0 | 216 | 96.4 | 424 | 96.6 | |
| Use of tobacco | 0,000 | ||||||||
| Yes | 74 | 49.7 | 44 | 55.0 | 62 | 27.7 | 180 | 39.7 | |
| No | 75 | 50.3 | 36 | 45.0 | 162 | 72.3 | 273 | 60.3 | |
| Changing sexual partners in the last three months | 0.000 | ||||||||
| Yes | 15 | 10.1 | 36 | 45.0 | 47 | 21.0 | 98 | 21.6 | |
| No | 134 | 89.9 | 44 | 55.0 | 177 | 79.0 | 355 | 78.4 | |
| Use of sexual accessories | 0.000 | ||||||||
| Yes | 51 | 34.2 | 19 | 23.8 | 22 | 9.8 | 92 | 20.3 | |
| No | 98 | 65.8 | 61 | 76.2 | 202 | 90.2 | 361 | 79.7 | |
| Vaginal penetration | 0.000 | ||||||||
| Yes | 129 | 86.6 | 77 | 96.3 | 224 | 100.0 | 430 | 94.9 | |
| No | 20 | 13.4 | 03 | 3.7 | 00 | 00 | 23 | 5.1 | |
| Anal penetration | 0.061 | ||||||||
| Yes | 24 | 16.1 | 20 | 25.0 | 59 | 26.3 | 103 | 22.7 | |
| No | 125 | 83.9 | 60 | 75.0 | 165 | 73.7 | 350 | 77.3 | |
| Oral sex | 0.208 | ||||||||
| Yes | 143 | 96.0 | 80 | 100.0 | 217 | 96.9 | 440 | 97.1 | |
| No | 06 | 4.0 | 00 | 00 | 07 | 3.1 | 13 | 2.9 | |
| Inconsistent use of condomc | 0.015 | ||||||||
| Yes | 26 | 17.4 | 07 | 8.7 | 52 | 23.2 | 85 | 18.8 | |
| No | 123 | 82.6 | 73 | 91.3 | 172 | 76.8 | 368 | 81.2 | |
| Vaginal douche | 0.015 | ||||||||
| Yes | 23 | 15.4 | 09 | 11.3 | 14 | 6.3 | 46 | 10.2 | |
| No | 126 | 84.6 | 71 | 88.7 | 210 | 93.7 | 407 | 89.8 | |
| HPV | 0.000 | ||||||||
| Positive | 59 | 39.6 | 54 | 67.5 | 129 | 57.6 | 242 | 53.4 | |
| Negative | 90 | 60.4 | 26 | 32.5 | 95 | 42.4 | 211 | 46.6 | |
| C. trachomatis | 0.606 | ||||||||
| Positive | 05 | 3.4 | 05 | 6.3 | 11 | 4.9 | 21 | 4.6 | |
| Negative | 144 | 96.6 | 75 | 93.7 | 213 | 95.1 | 432 | 95.4 | |
| Bacterial vaginosis | 0.013 | ||||||||
| Positive | 53 | 35.6 | 29 | 36.3 | 52 | 23.2 | 134 | 29.6 | |
| Negative | 96 | 64.4 | 51 | 63.7 | 172 | 76.8 | 319 | 70.4 | |
| Attend primary health care units | 0.000 | ||||||||
| Yes | 89 | 59.7 | 54 | 67.5 | 185 | 82.6 | 328 | 72.4 | |
| No | 60 | 40.3 | 26 | 32.5 | 39 | 17.4 | 125 | 27.6 | |
| Gynecological appointment in the last year | 0.001 | ||||||||
| Yes | 75 | 50.3 | 48 | 60.0 | 155 | 69.2 | 278 | 61.4 | |
| No | 74 | 49.7 | 32 | 40.0 | 69 | 30.8 | 175 | 38.6 | |
WSW, Women with homosexual practice; WSWM, Women with bisexual practice; WSM, Women with heterosexual practice; HPV, Human Papillomavirus.
The prevalence of bacterial vaginosis among the groups was: 35.6% WSW vs. 36.3% WSWM vs. 23.2% WSM; p = 0.013 (Table 1).
The significant differences between the groups were related to the frequency of some variables, such as: lives with a partner (26.8% WSW vs. 6.2% WSWM vs. 21.9% WSM; p = 0.001), remunerated activity (73.2% WSW vs. 46.2% WSWM vs. 66.5% WSM; p = 0.000), per capita family income (8.7% WSW vs. 10.0% WSWM vs. 3.6% WSM; p = 0.047), use of tobacco (49.7% WSW vs. 55.0% WSWM vs. 27.7% WSM; p = 0.000), changing sexual partners in the last three months (10.1% WSW vs. 45.0% WSWM vs. 21.0% WSM; p = 0.000), sexual accessories (34.2% WSW vs. 23.8% WSWM vs. 9.8% WSM; p = 0.000), receive vaginal penetration (86.6% WSW vs. 96.3% WSWM vs. 100.0% WSM; p = 0.000), consistent condom use (17.4% WSW vs. 8.7% WSWM vs. 23.2% WSM; p = 0.015), vaginal douching (15.4% WSW vs. 11.3% WSWM vs. 6.3% WSM; p = 0.015), HPV infection (39.6% WSW vs. 67.5% WSWM vs. 57.6% WSM; p = 0.000), attend primary health care units (59.7% WSW vs. 67.5% WSWM vs. 82.6% WSM; p = 0.000) and had a gynecological appointment in the last year (50.3% WSW vs. 60.0% WSWM vs. 69.2% WSM; p = 0.001) (Table 1).
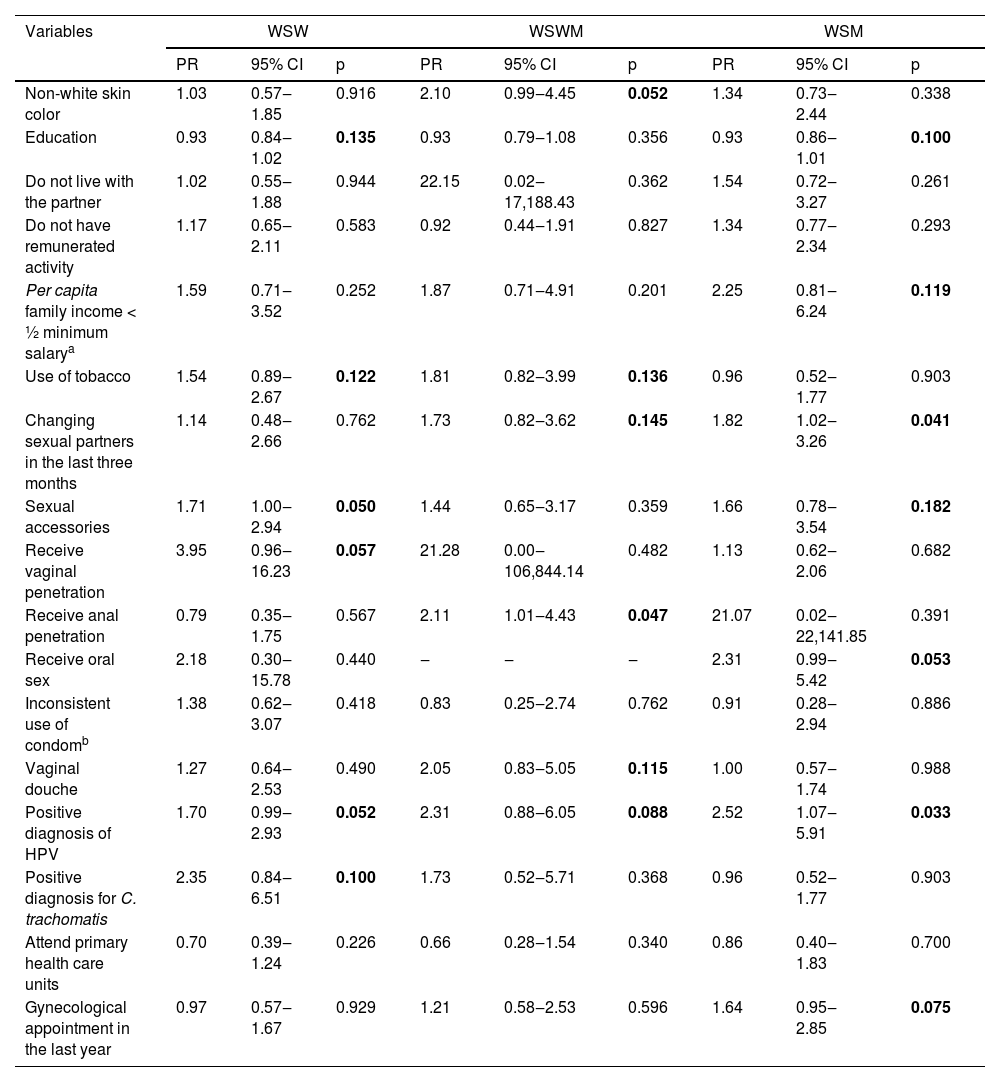
Considering the bivariate analyzes in the WSW group, the variables that presented p < 0.20 and were part of the multiple regression model were: years of education (0.93 [95% CI 0.84‒1.02]; p = 0.135), use of tobacco (1.54 [95% CI 0.89‒2.67]; p = 0.122), sexual accessories (1.71 [95% CI 1.00‒2.94]; p = 0.050), receive vaginal penetration (3.95 [95% CI 0.96‒16.23]; p = 0.057), positive diagnosis of HPV (1.70 [95% CI 0.99‒2.93]; p = 0.052) and positive diagnosis of C. trachomatis (2.35 [95% CI 0.84‒6.51); p = 0.100). In the WSWM group, the variables that were included in the multiple regression were: non-white ethnicity (2.10 [95% CI 0.99‒4.45]; p = 0.052); use of tobacco (1.81 [95% CI 0.82‒3.99]; p = 0.136), changing sexual partner in the last three months (1.73 [95% CI 0.82‒3.62]; p = 0.145), receive anal penetration (2.11 [95% CI 1.01‒4.43]; p = 0.047), vaginal douche (2.05 [95% CI 0.83‒5.05]; p = 0.115) and positive diagnosis of HPV (2.31 [95% CI 0.88‒6.05]; p = 0.088). Multiple regression in the WSM group included the variables: years of education (0.93 [95% CI 0.86‒1.01]; p = 0.100), per capita family income less than half the minimum salary (2.25 [95% CI 0.81‒6.24]; p = 0.119), changing sexual partner in the last three months (1.82 [95% CI 1.02‒3.26]; p = 0.041), sexual accessories (1.66 [95% CI 0.78‒3.54]; p = 0.182), inconsistent use of condoms (2.31 [95% CI 0.99‒5.42]; p = 0.053), positive diagnosis of C. trachomatis (2.52 [95% CI 1.07‒5.91]; p = 0.033) and did not have a gynecological appointment in the last year (1.64 [95% CI 0.95‒2.85]; p = 0.075) (Table 2).
Bivariate analysis for the association between bacterial vaginosis and demographic, behavioral and clinical variables between the studied groups.
| Variables | WSW | WSWM | WSM | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PR | 95% CI | p | PR | 95% CI | p | PR | 95% CI | p | |
| Non-white skin color | 1.03 | 0.57‒1.85 | 0.916 | 2.10 | 0.99‒4.45 | 0.052 | 1.34 | 0.73‒2.44 | 0.338 |
| Education | 0.93 | 0.84‒1.02 | 0.135 | 0.93 | 0.79‒1.08 | 0.356 | 0.93 | 0.86‒1.01 | 0.100 |
| Do not live with the partner | 1.02 | 0.55‒1.88 | 0.944 | 22.15 | 0.02‒17,188.43 | 0.362 | 1.54 | 0.72‒3.27 | 0.261 |
| Do not have remunerated activity | 1.17 | 0.65‒2.11 | 0.583 | 0.92 | 0.44‒1.91 | 0.827 | 1.34 | 0.77‒2.34 | 0.293 |
| Per capita family income < ½ minimum salarya | 1.59 | 0.71‒3.52 | 0.252 | 1.87 | 0.71‒4.91 | 0.201 | 2.25 | 0.81‒6.24 | 0.119 |
| Use of tobacco | 1.54 | 0.89‒2.67 | 0.122 | 1.81 | 0.82‒3.99 | 0.136 | 0.96 | 0.52‒1.77 | 0.903 |
| Changing sexual partners in the last three months | 1.14 | 0.48‒2.66 | 0.762 | 1.73 | 0.82‒3.62 | 0.145 | 1.82 | 1.02‒3.26 | 0.041 |
| Sexual accessories | 1.71 | 1.00‒2.94 | 0.050 | 1.44 | 0.65‒3.17 | 0.359 | 1.66 | 0.78‒3.54 | 0.182 |
| Receive vaginal penetration | 3.95 | 0.96‒16.23 | 0.057 | 21.28 | 0.00‒106,844.14 | 0.482 | 1.13 | 0.62‒2.06 | 0.682 |
| Receive anal penetration | 0.79 | 0.35‒1.75 | 0.567 | 2.11 | 1.01‒4.43 | 0.047 | 21.07 | 0.02‒22,141.85 | 0.391 |
| Receive oral sex | 2.18 | 0.30‒15.78 | 0.440 | ‒ | ‒ | ‒ | 2.31 | 0.99‒5.42 | 0.053 |
| Inconsistent use of condomb | 1.38 | 0.62‒3.07 | 0.418 | 0.83 | 0.25‒2.74 | 0.762 | 0.91 | 0.28‒2.94 | 0.886 |
| Vaginal douche | 1.27 | 0.64‒2.53 | 0.490 | 2.05 | 0.83‒5.05 | 0.115 | 1.00 | 0.57‒1.74 | 0.988 |
| Positive diagnosis of HPV | 1.70 | 0.99‒2.93 | 0.052 | 2.31 | 0.88‒6.05 | 0.088 | 2.52 | 1.07‒5.91 | 0.033 |
| Positive diagnosis for C. trachomatis | 2.35 | 0.84‒6.51 | 0.100 | 1.73 | 0.52‒5.71 | 0.368 | 0.96 | 0.52‒1.77 | 0.903 |
| Attend primary health care units | 0.70 | 0.39‒1.24 | 0.226 | 0.66 | 0.28‒1.54 | 0.340 | 0.86 | 0.40‒1.83 | 0.700 |
| Gynecological appointment in the last year | 0.97 | 0.57‒1.67 | 0.929 | 1.21 | 0.58‒2.53 | 0.596 | 1.64 | 0.95‒2.85 | 0.075 |
WSW, Women with homosexual practice; WSWM, Women with bisexual practice; WSM, Women with heterosexual practice; p, p-value; HPV, Human Papillomavirus; CI, Indicates Confidence Interval; PR, Prevalence Ratio.
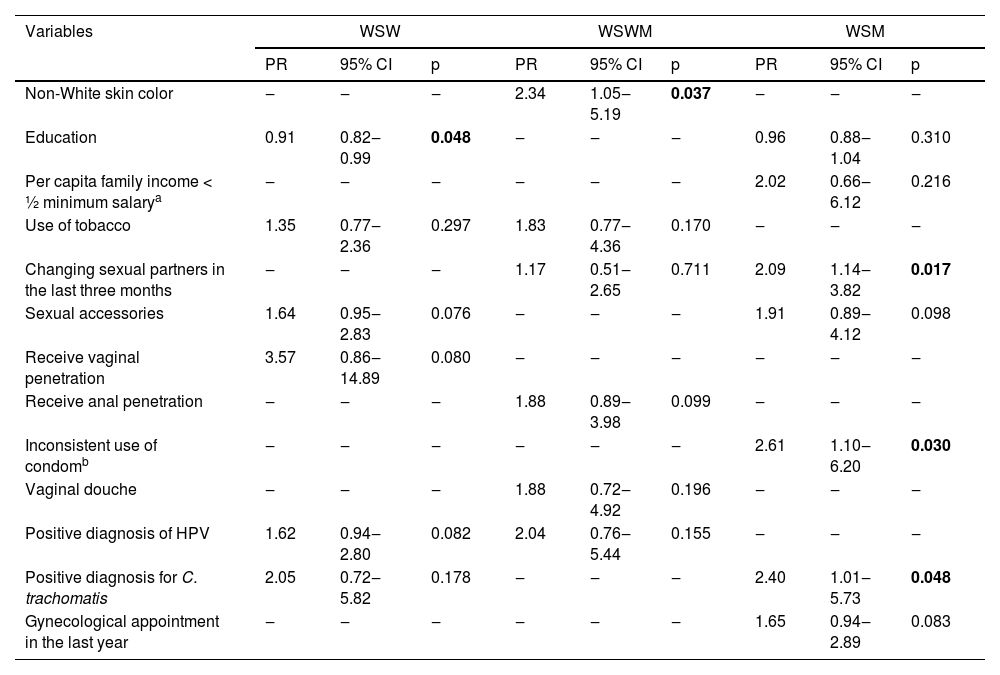
The multiple regressions that analyzed the factors associated with BV in the three groups studied are shown in Table 3.
Multiple regression that identified the factors associated with BV between the studied groups.
| Variables | WSW | WSWM | WSM | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PR | 95% CI | p | PR | 95% CI | p | PR | 95% CI | p | |
| Non-White skin color | ‒ | ‒ | ‒ | 2.34 | 1.05‒5.19 | 0.037 | ‒ | ‒ | ‒ |
| Education | 0.91 | 0.82‒0.99 | 0.048 | ‒ | ‒ | ‒ | 0.96 | 0.88‒1.04 | 0.310 |
| Per capita family income < ½ minimum salarya | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | 2.02 | 0.66‒6.12 | 0.216 |
| Use of tobacco | 1.35 | 0.77‒2.36 | 0.297 | 1.83 | 0.77‒4.36 | 0.170 | ‒ | ‒ | ‒ |
| Changing sexual partners in the last three months | ‒ | ‒ | ‒ | 1.17 | 0.51‒2.65 | 0.711 | 2.09 | 1.14‒3.82 | 0.017 |
| Sexual accessories | 1.64 | 0.95‒2.83 | 0.076 | ‒ | ‒ | ‒ | 1.91 | 0.89‒4.12 | 0.098 |
| Receive vaginal penetration | 3.57 | 0.86‒14.89 | 0.080 | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
| Receive anal penetration | ‒ | ‒ | ‒ | 1.88 | 0.89‒3.98 | 0.099 | ‒ | ‒ | ‒ |
| Inconsistent use of condomb | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | 2.61 | 1.10‒6.20 | 0.030 |
| Vaginal douche | ‒ | ‒ | ‒ | 1.88 | 0.72‒4.92 | 0.196 | ‒ | ‒ | ‒ |
| Positive diagnosis of HPV | 1.62 | 0.94‒2.80 | 0.082 | 2.04 | 0.76‒5.44 | 0.155 | ‒ | ‒ | ‒ |
| Positive diagnosis for C. trachomatis | 2.05 | 0.72‒5.82 | 0.178 | ‒ | ‒ | ‒ | 2.40 | 1.01‒5.73 | 0.048 |
| Gynecological appointment in the last year | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | 1.65 | 0.94‒2.89 | 0.083 |
WSW, Women with homosexual practice; WSWM, Women with bisexual practice; WSM, Women with heterosexual practice; p, p‒value; HPV, Human Papillomavirus; CI, Indicates Confidence Interval; PR, Prevalence Ratio.
Between the WSW, years of education was associated with protection against BV (0.91 [95% CI 0.82‒0.99]; p = 0.048). The prevalence of BV decreased by an average of 9.0% for each year of study completed by these women (Table 3).
Non-white skin color was independently associated with BV among WSWM, increasing the outcome prevalence by two and a half times (2.34 [95% CI 1.05‒5.19]; p = 0.037) (Table 3).
About the WSM, the factors associated with BV identified were: changing sexual partner in the last three months (2.09 [95% CI 1.14‒3.82]; p = 0.017), which increased twice the BV prevalence, do not use condoms consistently (2.61 [95% CI 1.10‒6.20]; p = 0.030) and positive diagnosis of C. trachomatis (2.40 [95% CI 1.01‒5.73]; p = 0.048) that increased the prevalence of the outcome by two and a half times (Table 3).
DiscussionThe present investigation identified that the factors associated with BV varied according to the types of sexual partnership experienced between the women included in this study. Among the WSM, the associated factors found were related to behavioral and clinical characteristics, and between the WSW and WSWM the factors were sociodemographic.
The education was independently associated with BV among WSW in this study, being a protective factor for this condition. Possibly, the highly educated WSW have a higher level of information and sociocultural conditions, what favors the reduction of the risk of having BV.16 No previous studies were found to present the level of education associated with BV among WSW.
Non-white skin color was identified as a factor associated with BV in the WSWM group. This relation was previously demonstrated in an English study, in 2004,10 which aimed to evaluate BV in lesbian and bisexual women. They found, among the associated factors, that non-Caucasian ethnicity increased the chances of BV among them.10 However, the authors considered their small sample size as a limitation of the study.
Ethnic and racial differences have been related as a predisposing factor to BV, but the reasons for this association remain unclear. A possible cause can be related to socioeconomic factors, limiting, and hampering the accessibility of non-white women to the health care system. A biological factor can be the lower number of protective lactobacilli.17-19
Among the WSM, the factors associated with BV were change the sexual partner in the last three months, inconsistent use of condom and positive diagnosis of C. trachomatis.
Although the pathogenesis of BV remains poorly understood,1,2 some studies have appointed a close association between sexual activity and BV.1 The possibility to transmit bacteria related to BV during sexual intercourse,20 and the consistent use of condoms as a protective management for this vaginal microbiota alteration7,21,22 were demonstrated previously, corroborating the findings of the present investigation.
A positive diagnosis for C. trachomatis was also independently associated with BV among the WSM. The association related to these two conditions can be harmful to these women, since C. trachomatis and Gardnerella vaginalis are etiologic agents of Pelvic Inflammatory Disease (PID), a condition that is responsible for serious inflammatory sequels, such as infertility, ectopic pregnancy and chronic pelvic pain.23 Thus, these women may also be exposed to important risks related to sexual and reproductive health, such as the transmission and acquisition of STIs, demonstrating the need for actions to ensure the reduction of this exposure, such as STI screening, educational activities for safe sex and condom distribution.
The divergence of factors associated with BV between the groups studied suggests the need of health professionals to provide assistance to sexual and reproductive health care to women in a non-prejudiced and stereotyped way, considering aspects of their sexuality,24 the type of sexual partners, because this condition can influence the risk to BV.
In the present study, we could also identify important characteristics of the groups. The WSW and WSWM showed a higher frequency of risk behaviors when compared to WSM, such as use of tobacco, sexual accessories, changing of sexual partners and low use of condom, conditions that may be related to the significantly higher prevalence of BV presented by these two groups. The highest prevalence of BV among WSW and WSWM when compared to WSM has already been demonstrated.25
The study has limitations regarding the sample size of the groups, which may have influenced the identification of other factors associated with BV among those investigated. However, its importance is emphasized, since it compares factors associated with BV in groups of women with homosexual, bisexual and heterosexual practices, and thus, increasing knowledge about the subject.
ConclusionsIn summary, the factors associated with BV were different among women with homosexual, bisexual and heterosexual practices. Women who had a higher risk of BV in the investigated sample were those with a lower educational level and non-white skin color among the groups with homosexual and bisexual practices, respectively. Related to women with heterosexual practices, the risk of BV was associated to changing sexual partners in the last three months, inconsistent use of condoms and have a positive diagnosis of C. trachomatis. Thus, the study suggests that health professionals investigate the type of sexual practice of women, avoiding heteronormative approaches.
FundingThis work was supported by Sao Paulo Research Foundation (FAPESP), grants: 2015/04,224–6 e 2018/19,649–0. MAOI is supported with a scholarship from Sao Paulo Research Foundation (FAPESP) Grant: 2018/14770–6. All other authors declare no conflict of interest.