The incidence of antimicrobial resistance is increasing in many parts of the world. The focus of this report is to examine changes in antimicrobial resistance epidemiology among clinical isolates of Enterobacterales and Pseudomonas aeruginosa collected in six Latin American countries as part of the Antimicrobial Testing Leadership and Surveillance (ATLAS) program from 2015 to 2020, with a focus on the in vitro activity of ceftazidime-avibactam against Multidrug-Resistant (MDR) isolates.
MethodsNon-duplicate, clinical isolates of Enterobacterales (n = 15,215) and P. aeruginosa (n = 4,614) collected by 40 laboratories in Argentina, Brazil, Chile, Colombia, Mexico, and Venezuela, from 2015 to 2020, underwent centralized Clinical Lab Standards Institute (CLSI) broth microdilution susceptibility testing. Minimum Inhibitory Concentration (MIC) values were interpreted using 2022 CLSI breakpoints. An MDR phenotype was defined by resistance to ≥ 3 of seven sentinel agents.
ResultsIn total, 23.3% of Enterobacterales and 25.1% of P. aeruginosa isolates were MDR. Annual percent MDR values for Enterobacterales were stable from 2015 to 2018 (21.3% to 23.7% year) but markedly increased in 2019 (31.5%) and 2020 (32.4%). Annual percent MDR values for P. aeruginosa were stable from 2015 to 2020 (23.0% to 27.6% year). Isolates were divided into two 3-year time-periods, 2015‒2017 and 2018‒2020, for additional analyses. For Enterobacterales, 99.3% of all isolates and 97.1% of MDR isolates from 2015‒2017 were ceftazidime-avibactam-susceptible compared to 97.2% and 89.3% of isolates, respectively, from 2018‒2020. For P. aeruginosa, 86.6% of all isolates and 53.9% of MDR isolates from 2015‒2017 were ceftazidime-avibactam-susceptible compared to 85.3% and 45.3% of isolates, respectively, from 2018‒2020. Among individual countries, Enterobacterales and P. aeruginosa collected in Venezuela showed the greatest reductions in ceftazidime-avibactam susceptibility over time.
ConclusionMDR Enterobacterales increased in Latin America from 22% in 2015 to 32% in 2020 while MDR P. aeruginosa remained constant at 25%. Ceftazidime-avibactam remains highly active against all clinical isolates of both Enterobacterales (97.2% susceptible, 2018‒2020) and P. aeruginosa (85.3%), and inhibited more MDR isolates (Enterobacterales, 89.3% susceptible, 2018‒2020; P. aeruginosa, 45.3%) than carbapenems, fluoroquinolones, and aminoglycosides.
The ATLAS (Antimicrobial Testing Leadership and Surveillance) global surveillance program tracks the in vitro activity of ceftazidime-avibactam and comparator antimicrobial agents against clinical isolates of Gram-negative bacilli associated with bloodstream, intraabdominal, respiratory tract, skin and soft tissue, and urinary tract infections in countries in Latin America, Africa, the Asia-Pacific region, Europe, the Middle East region, and North America.1 Previously published surveillance data describing Gram-negative bacilli from Latin American have provided only limited assessments of Multidrug-Resistant (MDR) isolates.2–7 Tracking the prevalence of, and changes in, MDR phenotypes is a critical component of surveillance initiatives as it not only identifies the contributions of shifts in resistance to individual, commonly prescribed empirical agents (e.g., cephalosporins, piperacillin-tazobactam, fluoroquinolones) to MDR phenotypes but also focuses attention on the activities of broad-spectrum agents, like carbapenems, and newer β-lactam/β-lactamase combination agents, like ceftazidime-avibactam, against emerging MDR phenotypes.
The global proliferation of ESBLs in Enterobacterales over the last decade resulted in increased clinical use of carbapenems and consequently, increased carbapenem and multidrug resistance. The World Health Organization (WHO) classifies carbapenem-resistant Enterobacterales and carbapenem-resistant Pseudomonas aeruginosa (most carbapenem-resistant isolates are also MDR) as critical, priority 1 pathogens8 and the United States Centers for Disease Control and Prevention (CDC) lists carbapenem-resistant Enterobacterales and MDR (frequently carbapenem-resistant) P. aeruginosa as urgent and serious threats.9 Development of new agents, such as β-lactam/β-lactamase inhibitor combinations, with activity against MDR and carbapenem-resistant Enterobacterales and P. aeruginosa, is critical, as is surveillance to monitor the ongoing performance of newer agents, such as ceftazidime-avibactam, as their clinical use increases. Ceftazidime-avibactam is a combination agent comprised of ceftazidime, a third-generation cephalosporin, and avibactam, a non-β-lactam β-lactamase-inhibitor. Avibactam inhibits Ambler class A, class C, and certain class D OXA-type β-lactamases but is inactive against Metallo-β-Lactamase (MBL) positive isolates.10
The present work intended to evaluate the in vitro activities of ceftazidime-avibactam and clinically relevant comparator agents against Gram-negative bacilli isolated from patients hospitalized in six Latin American countries from 2015 to 2020. We focused specific attention on the potential utility of ceftazidime-avibactam against organisms with MDR phenotypes divided into two three-year time periods, 2015‒2017 and 2018‒2020, to assess changes in antimicrobial susceptibility over time.
Materials and methodsBacterial isolatesIsolates of Gram-negative bacilli tested in the current study (n = 19,829) were collected as a part of the ATLAS global surveillance program from 2015 to 2020 by 40 medical center laboratory sites in six countries in Latin America: Argentina, Brazil, Chile, Colombia, Mexico, and Venezuela. Site participation within countries varied across the study years: Argentina (three sites/year), Brazil (five to eight sites/year), Chile (two or three sites/year), Colombia (two to six sites/year), Mexico (five to seven sites/year), and Venezuela (two or three sites/year). Twelve sites participated in all six years of the study; four sites participated for four or five years, and 24 sites participated for one to three years. Organisms were isolated from bloodstream (n = 3745), intraabdominal (n = 3101), respiratory tract (n = 4453), skin and soft tissue (n = 3786), urinary tract (n = 4740), and unspecified infection (n = 4) specimens and included 15,215 isolates of Enterobacterales and 4614 isolates of P. aeruginosa (Supplemental Table S1). Isolate identities were confirmed by IHMA (Schaumburg, IL, USA) using MALDI-TOF mass spectrometry (Bruker Daltonics, Billerica, MA, USA).
The ATLAS global surveillance program requests each participating medical center laboratory to collect annually defined quotas of isolates of selected bacterial species from patients with specific infection types and is not intended to evaluate the geographic prevalence of bacteria causing specific infection types. Isolates are limited to one per patient per year and are accepted independent of patient hospital location.
Antimicrobial susceptibility testingAntimicrobial susceptibility testing was performed in a central laboratory (IHMA) using the CLSI broth microdilution method.11 Avibactam was tested at a fixed concentration of 4 μg/mL. MICs were interpreted using 2022 CLSI M100 breakpoints.12 CLSI MIC breakpoints for ceftazidime-avibactam tested against isolates of Enterobacterales and P. aeruginosa are based on a dosage regimen of 2 g of ceftazidime plus 0.5 g of avibactam every 8 hours administered over 2 hours.12
The definition of an MDR phenotype included those isolates resistant to at least one agent in three or more relevant antimicrobial categories as per CLSI breakpoints. For both Enterobacterales and P. aeruginosa, the following categories (agents) were used to identify MDR isolates: aminoglycosides (amikacin), β-lactam/β-lactamase inhibitor combinations (piperacillin-tazobactam), carbapenems (meropenem), extended-spectrum cephalosporins (cefepime), fluoroquinolones (levofloxacin), monobactams (aztreonam), and polymyxins (colistin).
Ethical approvalEthical approval and informed consent were not required because all isolates received into the study followed multiple subcultures and were completely de-identified. The secondary research use of de-identified isolates is considered exempt research according to the Regulations for the Protection of Human Subjects in Research of the U.S. Department of Health and Human Services, Office for Human Research Protections (45 CFR 46).
ResultsMDR phenotypes were present in 23.3% of 15,215 Enterobacterales isolates and 25.1% of 4614 P aeruginosa isolates tested from six Latin American countries from 2015 to 2020 (Table 1). The annual percent MDR values for Enterobacterales were stable from 2015 to 2018 (21.3 to 23.7% year) but demonstrated a marked increase in 2019 (31.5%) and 2020 (32.4%). The annual percent MDR values for P. aeruginosa fluctuated randomly over a narrow range from 2015 to 2020 (23.0% to 27.6% year) and did not demonstrate an increasing or decreasing trend.
Percentage of Enterobacterales and P. aeruginosa isolates from Latin America with MDR phenotypes stratified by year.
| % MDRa | |||||||
|---|---|---|---|---|---|---|---|
| 2015‒2020 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | |
| Enterobacterales (n = 15,215) | 23.3% | 21.7% | 22.8% | 21.3% | 23.7% | 31.5% | 32.4% |
| P. aeruginosa (n = 4614) | 25.1% | 27.6% | 26.0% | 23.0% | 25.2% | 26.1% | 23.5% |
An MDR phenotype was defined as resistant to ≥ 3 sentinel agents, including: amikacin, aztreonam, cefepime, colistin, levofloxacin, meropenem, and piperacillin-tazobactam. The n for Enterobacterales/P. aeruginosa by year was: 2015, 2353/707; 2016, 2662/665; 2017, 2797/692; 2018, 2255/583; 2019, 2428/912; and 2020, 2720/1055.
Differences in percent susceptible values over time, for each of the 10 antimicrobial agents tested, was assessed by dividing isolates of Enterobacterales and P. aeruginosa into two 3-year time-periods, 2015‒2017 and 2018‒2020. The ceftazidime-avibactam percent susceptible value for all Enterobacterales isolates decreased minimally (by 2.1%) when 2015‒2017 isolates (99.3% susceptible) were compared with 2018‒2020 isolates (97.2% susceptible) (Table 2). All comparator agents tested also showed a percent susceptible value decrease from 2015‒2017 to 2018‒2020, ranging from 1.7% for amikacin to 6.5% for cefepime. Imipenem and meropenem percent susceptible values decreased by 4.5% (from 84.6% to 80.1%) and 4.2% (from 94.6% to 90.4%), respectively.
In vitro susceptibility of Enterobacterales collected in six Latin American countriesa divided into two 3-year time periods 2015‒2017 and 2018‒2020.
| Collection years (n) | Compound | Range | MIC50 | MIC90 | CLSI Interpretationb | ||
|---|---|---|---|---|---|---|---|
| (µg/mL) | %S | %I | %R | ||||
| 2015‒2017 (7736) | Amikacin | ≤0.25 – >32 | 2 | 8 | 96.5 | 1.6 | 1.8 |
| Aztreonam | ≤0.015 – >128 | 0.12 | 128 | 69.2 | 1.9 | 28.9 | |
| Cefepime | ≤0.12 – >16 | ≤0.12 | >16 | 71.0 | 5.5 | 23.4 | |
| Ceftazidime | ≤0.015 – >128 | 0.25 | 64 | 70.9 | 3.3 | 25.9 | |
| Ceftazidime-avibactam | ≤0.015 – >128 | 0.12 | 0.5 | 99.3 | NA | 0.7 | |
| Colistin | ≤0.06 – >8 | 0.25 | >8 | NA | 83.0 | 17.0 | |
| Imipenem | ≤0.03 – >8 | 0.25 | 2 | 84.6 | 7.5 | 7.8 | |
| Levofloxacin | ≤0.004 – >8 | 0.25 | >8 | 61.1 | 5.5 | 33.4 | |
| Meropenem | ≤0.004 – >8 | 0.03 | 0.12 | 94.6 | 0.8 | 4.6 | |
| Piperacillin Tazobactam | ≤0.25 – >128 | 4 | 128 | 77.9 | 4.3 | 17.8 | |
| 2018‒2020 (7479) | Amikacin | ≤0.25 – >64 | 2 | 8 | 94.8 | 2.3 | 2.9 |
| Aztreonam | ≤0.015 – >128 | 0.12 | >64 | 63.6 | 2.7 | 33.7 | |
| Cefepime | ≤0.12 – >32 | ≤0.12 | >32 | 64.5 | 7.7 | 27.7 | |
| Ceftazidime | ≤0.015 – >128 | 0.5 | >64 | 64.5 | 3.7 | 31.8 | |
| Ceftazidime-avibactam | ≤0.015 – >128 | 0.12 | 1 | 97.2 | NA | 2.8 | |
| Colistin | ≤0.06 – >8 | 0.5 | >8 | NA | 80.2 | 19.8 | |
| Imipenem | ≤0.06 – >8 | 0.25 | 4 | 80.1 | 6.6 | 13.3 | |
| Levofloxacin | ≤0.25 – >8 | 0.5 | >8 | 58.8 | 6.6 | 34.5 | |
| Meropenem | ≤0.06 – >16 | ≤0.06 | 1 | 90.4 | 1.0 | 8.6 | |
| Piperacillin Tazobactam | ≤0.12 – >64 | 2 | >64 | 73.8 | 5.5 | 20.7 | |
Among all P. aeruginosa isolates tested, changes in percent susceptible values for all study agents were minimal and random across the two time-periods (2015‒2017 and 2018‒2020), ranging from an increase in the percent susceptible value of 3.4% for aztreonam from 2015‒2017 to 2018‒2020 to a decrease of 3.2% for imipenem over the same time period (Table 3). The ceftazidime-avibactam percent susceptible value for all P. aeruginosa isolates tested only decreased by 1.3% for isolates from 2015‒2017 (86.6% susceptible) compared with 2018‒2020 isolates (85.3% susceptible).
. In vitro susceptibility of P. aeruginsoa collected in six Latin American countriesa divided into two 3-year time periods 2015‒2017 and 2018‒2020.
| Collection years (n) | Compound | Range | MIC50 | MIC90 | CLSI Interpretationb | ||
|---|---|---|---|---|---|---|---|
| (µg/mL) | %S | %I | %R | ||||
| 2015‒2017 (2054) | Amikacin | ≤ 0.25 – > 32 | 4 | > 32 | 82.0 | 3.4 | 14.6 |
| Aztreonam | 0.06 – > 128 | 8 | 64 | 58.9 | 13.9 | 27.2 | |
| Cefepime | 0.5 – > 16 | 4 | > 16 | 72.5 | 10.7 | 16.8 | |
| Ceftazidime | 0.06 – > 128 | 4 | 128 | 69.2 | 4.7 | 26.1 | |
| Ceftazidime-avibactam | 0.03 – > 128 | 2 | 32 | 86.6 | NA | 13.4 | |
| Colistin | ≤ 0.06 – > 8 | 1 | 2 | NA | 99.3 | 0.7 | |
| Imipenem | 0.12 – > 8 | 2 | > 8 | 59.9 | 5.8 | 34.2 | |
| Levofloxacin | 0.015 – > 8 | 1 | > 8 | 59.0 | 7.5 | 33.5 | |
| Meropenem | ≤ 0.004 – > 8 | 1 | > 8 | 65.0 | 5.7 | 29.3 | |
| Piperacillin Tazobactam | ≤ 0.25 – > 128 | 8 | > 128 | 66.2 | 14.4 | 19.4 | |
| 2018‒2020 (2560) | Amikacin | ≤ 0.25 – > 64 | 4 | 64 | 81.1 | 3.1 | 15.8 |
| Aztreonam | 0.12 – > 128 | 8 | 64 | 62.3 | 14.1 | 23.6 | |
| Cefepime | ≤ 0.12 – > 32 | 4 | > 32 | 71.3 | 10.7 | 17.9 | |
| Ceftazidime | 0.25 – > 128 | 4 | 64 | 70.9 | 4.4 | 24.6 | |
| Ceftazidime-avibactam | ≤ 0.03 – > 128 | 2 | 32 | 85.3 | NA | 14.7 | |
| Colistin | ≤ 0.12 – > 8 | 1 | 2 | NA | 99.8 | 0.2 | |
| Imipenem | 0.12 – > 8 | 2 | >8 | 56.7 | 8.9 | 34.4 | |
| Levofloxacin | ≤ 0.25 – > 8 | 0.5 | >8 | 62.2 | 8.1 | 29.7 | |
| Meropenem | ≤ 0.06 – > 16 | 1 | >16 | 65.0 | 6.4 | 28.6 | |
| Piperacillin Tazobactam | ≤ 0.12 – > 64 | 8 | >64 | 67.5 | 14.3 | 18.3 | |
For the subset of MDR Enterobacterales isolates, 97.1% from 2015‒2017 were ceftazidime-avibactam-susceptible, 7.8% higher than isolates from 2018‒2020 (89.3% susceptible) (Table 4). Amikacin, imipenem, and meropenem percent susceptible values decreased 5.0%, 14.2%, and 14.2%, respectively, when 2015‒2017 and 2018‒2020 isolates were compared. Amikacin, imipenem, and meropenem percent susceptible values were 81.2%, 60.6%, and 62.2%, respectively, for 2018‒2020 isolates of MDR Enterobacterales.
. In vitro susceptibility of multidrug-resistant Enterobacterales collected in six Latin American countriesa divided into two 3-year time periods 2015‒2017 and 2018‒2020.
| Collection years (n) | Compound | Range | MIC50 | MIC90 | CLSI Interpretationb | ||
|---|---|---|---|---|---|---|---|
| (µg/mL) | %S | %I | %R | ||||
| 2015‒2017 (1701) | Amikacin | ≤ 0.25 – > 32 | 4 | 32 | 86.2 | 6.0 | 7.8 |
| Aztreonam | ≤ 0.015 – > 128 | 64 | > 128 | 3.5 | 0.6 | 95.9 | |
| Cefepime | ≤ 0.12 – > 16 | > 16 | > 16 | 3.4 | 4.7 | 91.9 | |
| Ceftazidime | 0.03 – > 128 | 32 | > 128 | 6.8 | 5.6 | 87.5 | |
| Ceftazidime-avibactam | ≤ 0.015 – > 128 | 0.25 | 2 | 97.1 | NA | 2.9 | |
| Colistin | ≤ 0.06 – > 8 | 0.5 | > 8 | NA | 87.3 | 12.7 | |
| Imipenem | 0.06 – > 8 | 0.25 | > 8 | 74.8 | 3.1 | 22.2 | |
| Levofloxacin | ≤ 0.03 – > 8 | > 8 | > 8 | 8.1 | 4.4 | 87.5 | |
| Meropenem | 0.015 – > 8 | 0.06 | > 8 | 76.4 | 2.9 | 20.7 | |
| Piperacillin Tazobactam | ≤ 0.25 – > 128 | 32 | >128 | 32.2 | 8.5 | 59.3 | |
| 2018‒2020 (1838) | Amikacin | 0.5 – > 64 | 4 | 64 | 81.2 | 7.8 | 11.0 |
| Aztreonam | ≤ 0.015 – > 128 | > 64 | > 128 | 5.1 | 0.7 | 94.2 | |
| Cefepime | ≤ 0.12 – > 32 | >32 | > 32 | 2.2 | 4.9 | 92.9 | |
| Ceftazidime | 0.12 – > 128 | 64 | > 128 | 5.4 | 3.6 | 91.0 | |
| Ceftazidime-avibactam | ≤ 0.015 – > 128 | 0.5 | > 64 | 89.3 | NA | 10.7 | |
| Colistin | ≤ 0.06 – > 8 | 0.5 | > 8 | NA | 82.6 | 17.4 | |
| Imipenem | ≤ 0.06 – > 8 | 0.5 | > 8 | 60.6 | 3.2 | 36.2 | |
| Levofloxacin | ≤ 0.25 – > 8 | >8 | > 8 | 8.4 | 6.0 | 85.6 | |
| Meropenem | ≤ 0.06 – > 16 | 0.12 | > 16 | 62.2 | 3.3 | 34.5 | |
| Piperacillin Tazobactam | 0.25 – > 64 | > 64 | > 64 | 26.1 | 8.5 | 65.3 | |
Against MDR P. aeruginosa isolates, ceftazidime-avibactam was the most active antimicrobial agent tested, inhibiting 53.9% and 45.3% of 2015‒2017 and 2018‒2020 isolates, respectively (Table 5). The amikacin percent susceptible value decreased by 9.4% for 2015‒2017 isolates (43.5% susceptible) compared to 2018‒2020 isolates (34.1% susceptible). Percent susceptible values for imipenem, meropenem, and all other agents tested against 2018‒2020 isolates of MDR P. aeruginosa were ≤ 12.0%.
In vitro susceptibility of isolates of multidrug-resistant P. aeruginosa collected in six Latin American countriesa divided into two 3-year time periods 2015‒2017 and 2018‒2020.
| Collection years (n) | Compound | Range | MIC50 | MIC90 | CLSI Interpretationb | ||
|---|---|---|---|---|---|---|---|
| (µg/mL) | %S | %I | %R | ||||
| 2015‒2017 (527) | Amikacin | 0.5 – > 32 | > 32 | > 32 | 43.5 | 6.3 | 50.3 |
| Aztreonam | 0.25 – > 128 | 64 | > 128 | 7.6 | 13.7 | 78.7 | |
| Cefepime | 4 – > 16 | > 16 | > 16 | 11.8 | 24.7 | 63.6 | |
| Ceftazidime | 0.25 – > 128 | 64 | > 128 | 15.2 | 6.3 | 78.6 | |
| Ceftazidime-avibactam | 0.25 – >128 | 8 | 64 | 53.9 | 0.0 | 46.1 | |
| Colistin | 0.12 – 8 | 1 | 2 | NA | 98.7 | 1.3 | |
| Imipenem | 0.25 – >8 | >8 | >8 | 12.7 | 1.3 | 86.0 | |
| Levofloxacin | 0.25 – > 8 | > 8 | > 8 | 8.3 | 4.0 | 87.7 | |
| Meropenem | 0.12 – > 8 | > 8 | > 8 | 8.2 | 4.9 | 86.9 | |
| Piperacillin Tazobactam | 4 – > 128 | > 128 | > 128 | 9.5 | 26.0 | 64.5 | |
| 2018‒2020 (633) | Amikacin | ≤ 0.25 – > 64 | 64 | > 64 | 34.1 | 7.0 | 58.9 |
| Aztreonam | 0.25 – > 128 | 32 | > 128 | 11.5 | 15.6 | 72.8 | |
| Cefepime | 4 – > 32 | 32 | > 32 | 6.0 | 23.4 | 70.6 | |
| Ceftazidime | 1 – > 128 | 64 | > 128 | 10.6 | 7.6 | 81.8 | |
| Ceftazidime-avibactam | 0.5 – > 128 | 16 | > 64 | 45.3 | 0.0 | 54.7 | |
| Colistin | ≤ 0.12 – > 8 | 1 | 2 | NA | 99.7 | 0.3 | |
| Imipenem | 1 – > 8 | > 8 | > 8 | 12.0 | 4.4 | 83.6 | |
| Levofloxacin | ≤ 0.25 – > 8 | > 8 | > 8 | 8.5 | 3.5 | 88.0 | |
| Meropenem | 0.12 – > 16 | > 16 | > 16 | 9.0 | 6.0 | 85.0 | |
| Piperacillin Tazobactam | ≤ 0.12 – > 64 | >64 | >64 | 4.9 | 30.6 | 64.5 | |
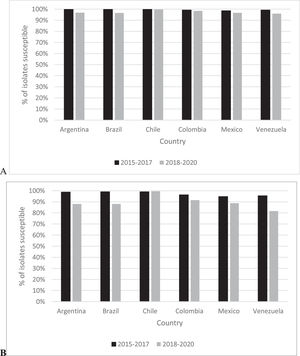
Fig. 1 provides country-specific ceftazidime-avibactam percent susceptible values for all Enterobacterales isolates and for the MDR isolate subset divided into 2015‒2017 and 2018‒2020 time periods. In 2015‒2017, the range of ceftazidime-avibactam percent susceptible values for all Enterobacterales isolates was 98.6% (Mexico) to 99.8% (Brazil, Chile) compared to a range of 96.0% (Venezuela) to 99.8% (Chile) in 2018‒2020. Among all Enterobacterales isolates collected, susceptibility to ceftazidime-avibactam was unchanged over time (< 2% difference) for isolates from Chile, Colombia, and Mexico while the percent susceptible value for isolates from Argentina, Brazil, and Venezuela decreased marginally from 2015‒2017 to 2018‒2020, by 2.9, 3.3%, and 3.2%, respectively. In 2015‒2017, 94.7% (Mexico) to 99.1% (Brazil, Chile) of MDR Enterobacterales isolates collected were ceftazidime-avibactam-susceptible compared to 81.6% (Venezuela) to 99.5% (Chile) of MDR Enterobacterales isolates from 2018‒2020. MDR Enterobacterales isolates from Venezuela (13.9% difference), Brazil (11.0%), Argentina (10.7%), Mexico (5.9%), and Colombia (4.8%) demonstrated decreased percent susceptible values from 2015‒2017 to 2018‒2020 while MDR Enterobacterales collected in Chile remained unchanged.
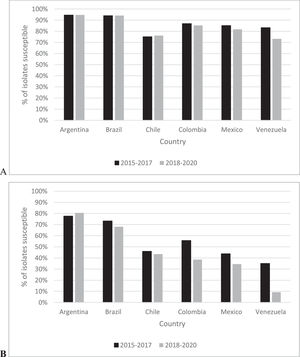
For all P. aeruginosa isolates, the range of ceftazidime-avibactam percent susceptible values by country was 75.0% (Chile) to 94.5% (Argentina) in 2015‒2017 compared to 73.1% (Venezuela) to 94.6% (Argentina) for isolates from 2018‒2020 (Fig. 2). Among all P. aeruginosa isolates collected, susceptibility to ceftazidime-avibactam was unchanged (< 2% difference) for isolates from Argentina, Brazil, Chile, and Colombia; the percent susceptible value for isolates from Mexico and Venezuela decreased by 3.4% and 10.1%, respectively, from 2015‒2017 to 2018‒2020. In 2015‒2017, 35.1% (Venezuela) to 77.6% (Argentina) of MDR P. aeruginosa isolates collected were ceftazidime-avibactam-susceptible compared to 9.2% (Venezuela) to 80.5% (Argentina) in 2018‒2020. MDR P. aeruginosa isolates from Argentina remained unchanged from 2015‒2017 to 2018‒2020 while isolates from Venezuela (25.9%), Colombia (17.1%), Mexico (9.3%), Brazil (5.3% decrease), and Chile (2.5%) demonstrated decreased percent susceptible values over time.
DiscussionIt is widely agreed that the prevalence of MDR Gram-negative bacilli is increasing worldwide and that these pathogens constitute a global threat to public health as they are associated with delays in initiation of, or the absence of, adequate antimicrobial therapy; increased morbidity, mortality, and lower cure rates; as well as increased length of hospital stays and hospital costs.13–15 Unfortunately, to date, a universal, harmonized definition of MDR has not been published. Publications describing MDR pathogens frequently define MDR using the proposed interim international definitions published by Magiorakos and others, in 2012.16 These definitions have never been revised and are not a definitive standard. Clearly establishing, applying, and communicating a consistent definition of MDR is essential to support resistance tracking, and infection control and antimicrobial stewardship initiatives. Using a consistent definition of MDR, defined using prescribed, pathogen-specific agents, is currently the best mechanism to track changes in MDR prevalence. Attention should always be paid to the definition of MDR used by study authors given its inherent flexibility.
In the current study, MDR, was defined by resistance (using CLSI breakpoints)12 to three or more antimicrobial categorical markers (amikacin, aztreonam, cefepime, colistin, levofloxacin, meropenem, piperacillin-tazobactam) and was present in 23.3% of all Enterobacterales and 25.1% of all P. aeruginosa isolates tested from 2015 to 2020 (Table 1). An earlier published estimate of MDR in Enterobacterales isolates collected in 2012‒2015, using a similar panel of defining antimicrobial agents, against isolates from the same six Latin American countries, reported 20.8% of 7665 Enterobacterales isolates to be MDR with percent MDR/country ranging from 16.3% of isolates from Venezuela to 24.2% of isolates from Chile.2 The same study also reported that 98.8% of all MDR isolates were ceftazidime-avibactam-susceptible and that ≥ 97.7% of MDR isolates collected in each of the six countries tested were ceftazidime-avibactam-susceptible,2 values higher than in the current study (overall, 89.3% of MDR Enterobacterales from 2018‒2020 were ceftazidime-avibactam-susceptible with a range by country of 81.6% [Venezuela] to 99.5% [Chile]) (Fig. 1).
Regardless of the high numbers of MDR isolates (23.3%) in the current study, all clinical isolates of Enterobacterales from the six Latin American countries studied were highly susceptible to ceftazidime-avibactam, even though the ceftazidime-avibactam percent susceptible value decreased (by 2.1%) from 99.3% for 2015‒2017 isolates to 97.2% for 2018‒2020 isolates (Table 2). All comparator agents tested also showed percent susceptible value decreases of up to 6.5% (cefepime) from 2015‒2017 to 2018‒2020. Imipenem and meropenem percent susceptible values decreased by 4.5% (from 84.6% to 80.1%) and 4.2% (from 94.6% to 90.4%), respectively. A comparative 2019 study of 1161 Enterobacterales isolates from similar Latin American countries reported that 29.6% of isolates were MDR and percent susceptible values for amikacin, levofloxacin, and meropenem were 92.0%, 60.0%, and 94.4%, respectively.6 Imipenem and meropenem percent susceptible values in 2018‒2020 (current study) were as much as 10% lower than values reported for Enterobacterales isolates collected in 2004‒2010 from identical countries.5
Current study results for ceftazidime-avibactam align closely with those of previous studies of clinical isolates of Enterobacterales, collected between the years 2012 and 2019, from the same six countries. Each study reported >98% of isolates as ceftazidime-avibactam-susceptible.2-4,7 The changes in susceptibility to ceftazidime-avibactam seem to correlate with increases in the incidence of MBLs in Latin American isolates over the same time period: 0.2% of isolates collected in 2012‒2015 were MBL-positive;2 as were 0.6% of isolates collected in 2015−20173 and 1.3% of isolates collected in 2017‒2019.4 MBLs increased more than two-fold for isolates collected in Colombia and Venezuela in a 2017‒2019 report4 compared to a an earlier 2012‒2015 report.2
The increase in MDR Enterobacterales identified in the years 2019 (31.5%) and 2020 (32.4%) in the current study (Table 1) align closely with decreases in carbapenem susceptibility (14.2% decrease in susceptibility to both imipenem and meropenem) and ceftazidime-avibactam susceptibility (7.8% decrease) (Table 4). Medical center laboratory site changes over time could not be linked to the increased MDR Enterobacterales observed in 2019 and 2020. Resistance to carbapenems among Enterobacterales is generally mediated by β-lactamase production.15 Isolates carrying carbapenemases often demonstrate MDR phenotypes that further limit therapeutic options. Carbapenemase-producing, MBL-negative Enterobacterales isolates collected in Latin America between 2016 and 2018 were reported to be uniformly susceptible to ceftazidime-avibactam.17 The activity of ceftazidime-avibactam against carbapenem-resistant Enterobacterales (many of these isolates are also MDR) depends upon the carbapenemase present (serine carbapenemase vs. MBL) as ceftazidime-avibactam is inactive against MBLs.17 In the current study, the decreases in susceptibility to carbapenems and ceftazidime-avibactam suggests increased presence of carbapenemases in 2019 and 2020, including MBLs that would confer resistance to all of these agents. This finding requires deeper study to determine a precise mechanism and necessitates continued monitoring. Fig. 1 suggests that MBLs may be emerging, or may have emerged in Argentina, Brazil, and Venezuela given the lowered ceftazidime-avibactam percent susceptible values among MDR isolates in 2018‒2020. A recent study reported that >50% of meropenem-nonsusceptible Enterobacterales collected in Venezuela from 2017 to 2019 carried NDM or VIM; the same study also documented MBL carriage in isolates from Argentina, Brazil, Colombia, and Mexico, but not Chile.4 Only 11% of carbapenem-nonsusceptible Enterobacterales from Venezuela carried an MBL in a 2012‒2015 study.2 Comparing the same two studies also demonstrated an overall increase in MBLs in Enterobacterales isolates from all six Latin American countries from 6% of carbapenem-nonsusceptible Enterobacterales in 2012‒20152 to > 25% of isolates in the 2017‒2019.2
In the study of 2012‒2015 isolates mentioned earlier for Enterobacterales, an MDR phenotype was also present in 25.3% (of 1794) of P. aeruginosa isolates with percentages of isolates testing as MDR ranging from 20.4% (Mexico) to 34.0% (Chile).2 Of these MDR isolates, 57.1% were ceftazidime-avibactam-susceptible. In that study, ceftazidime-avibactam was least active against MDR isolates from Venezuela (37.3% susceptible) and Mexico (45.2% susceptible), and it was most active against MDR isolates from Argentina (77.6% susceptible) and Brazil (72.4% susceptible).
In the current study, changes in the percent susceptible values for all agents tested against all P. aeruginosa isolates between the two time-periods (2015‒2017 and 2018‒2020) were minimal (Table 3). Imipenem and meropenem percent susceptible values in 2018‒2020 (current study) were similar to values reported for P. aeruginosa isolates collected in 2004‒2010 from identical countries.5 The ceftazidime-avibactam percent susceptible value for all P. aeruginosa isolates tested decreased from 86.6% for 2015‒2017 isolates to 85.3% for 2018‒2020 isolates (1.3% difference). Previous studies that tested clinical isolates of P. aeruginosa, from the same six countries that were included in the current study, against ceftazidime-avibactam, reported results similar to ours: 87.4% of isolates collected in 2012‒2015,2 86.6% of isolates collected in 2015–2017,3 and 86.9% of isolates from 2017‒20194 were ceftazidime-avibactam-susceptible. The percent susceptibility to ceftazidime-avibactam increased to 92.8% when only MBL-negative isolates of P. aeruginosa were considered in the 2012‒2015 study.2
Based on data from 1997 to 2017, other authors have reported that the frequency of MDR isolates among P. aeruginosa in Latin America is not increasing.3 However, the composition of MDR isolates appears to be changing, particularly in Venezuela, Colombia, and Mexico, based on the current study. We also did not observe a directional change in the annual percentage of P. aeruginosa isolates with MDR phenotypes from 2015 to 2020. However, we did see a 9.4% decrease in the amikacin percent susceptible value and an 8.6% decrease in the ceftazidime-avibactam percent susceptible value when 2015‒2017 and 2018‒2020 isolates were compared (Table 5). Given that the vast majority of MDR P. aeruginosa from 2015‒2017 were already carbapenem-resistant (approximately 85% of isolates), a decreased ceftazidime-avibactam-susceptible percent value in 2018‒2020 may again indicate increased presence of MBLs. Fig. 2 suggests that the presence of MBLs in P. aeruginosa, particularly MDR P. aeruginosa, continue to increase and is of greatest concern in Venezuela, Colombia, and Mexico. A recent study reported that almost 70% of meropenem-nonsusceptible P. aeruginosa collected in Venezuela from 2017 to 2019 carried the VIM MBL; the same study also documented MBL carriage in meropenem-nonsusceptible P. aeruginosa isolates from Argentina, Brazil, Colombia, Chile, and Mexico.4 An increase in the presence of MBLs among carbapenem-nonsusceptible Enterobacterales from 15% of isolates in 2012‒20154 to 27% of isolates in 2017‒20192 in same six Latin American countries has been reported. In another study, unexpectedly, greater than 50% (53.2%) of carbapenemase-producing, MBL-negative P. aeruginosa isolates collected in Latin America between 2016 and 2018 were ceftazidime-avibactam-resistant.17 The mechanism underlying this observation remains cryptic and may involve undetected serine carbapenemase variants and/or another mechanism.17 Continued monitoring of MBL increases appears mandatory for both P. aeruginosa and Enterobacterales, as ceftazidime-avibactam constitutes a last-line agent and the emergence of antimicrobial resistance among Gram-negative pathogens continues to outpace the development, distribution, and global availability of new agents.
There are two primary limitations to this study. First, the ATLAS global surveillance program collects predefined numbers of isolates of each species from a limited number of laboratories per country, therefore, the data generated cannot be extrapolated to represent all isolates within a country and test results may over- or under-represent true rates of antimicrobial susceptibility. Second, there was some variability in annual medical center laboratory site participation over the duration of the study, which may impact the results. Of the 40 sites that participated from 2015 to 2020, only 12 (30%) participated in all 6 years.
In conclusion, MDR phenotypes currently account for approximately 25% of clinical isolates of both Enterobacterales and P. aeruginosa in Latin American countries. Ceftazidime-avibactam continued to be highly active against both Enterobacterales (> 97% of isolates susceptible) and P. aeruginosa (>85% of isolates susceptible) from Latin America in 2018‒2020. In 2018‒2020, differences in ceftazidime-avibactam percent susceptible values by country were more pronounced for all isolates and MDR isolates of P. aeruginosa (73.1%‒94.6%; 9.2%‒80.5%) than for Enterobacterales (96.0%‒99.8%; 81.6%‒99.5%) (Fig. 1 and Fig. 2). Given ceftazidime-avibactam's current role in the treatment of Gram-negative infections, that is, as treatment for patients infected with MDR and/or carbapenem-nonsusceptible isolates, antimicrobial susceptibility testing of ceftazidime-avibactam is encouraged concurrent with therapy initiation, as resistance to this agent appears to be increasing in some countries. In this regard, continued surveillance of the activity of ceftazidime-avibactam, and as well as alternative agents, is crucial to monitor ongoing activity and to identify further changes as they occur.
FundingFunding for this research, which was performed at IHMA and included compensation for services related to preparing this manuscript, was provided by Pfizer, Inc. The sponsor participated in the development of the overall study design, but collection and testing of isolates, data analysis and manuscript preparation were independently performed by IHMA.
The authors thank all ATLAS participants for their contributions to the program.