Tuberculosis (TB) is still considered a major global public health problem in the world and there is a concern about the worldwide increase of drug-resistance (DR). This paper describes the analysis of three Mycobacterium tuberculosis isolates from a single patient collected over a long treatment period of time. DR was initially investigated through phenotypic testing, followed by line probe assays (LPAs) and whole genome sequencing (WGS). It presents an intriguing situation where a multidrug-resistant (MDR-) TB case was diagnosed and treated based only on late phenotypic drug susceptibility testing of isolate 1. During the treatment, another two isolates were cultivated: isolate 2, nine months after starting MDR-TB treatment; and isolate 3, cultivated five months later, during regular use of anti-TB drugs. These two isolates were evaluated using molecular LPA and WGS, retrospectively. All mutations detected by LPA were also detected in the WGS, including conversion from fluoroquinolones susceptibility to resistance from isolate 2 to isolate 3. WGS showed additional mutations, including some which may confer resistance to other drugs not tested (terizidone/cycloserine) and mutations with no correspondent resistance in drug susceptibility testing (streptomycin and second-line injectable drugs).
Tuberculosis (TB) is still considered a major global public health problem, and Brazil is the 16th country in absolute number of cases. Despite consistent advances achieved with control measures, there are still major challenges to face the growing resistance to anti-tuberculosis drugs in several countries, including Brazil.1 Molecular epidemiology studies of M. tuberculosis have gained emphasis among clinical researchers. New knowledge on the TB pathogenesis and its causative agent could be the key to the development of new control strategies.2,3 Recently, the World Health Organization set the Global “Stop TB Plan” to find patients harboring resistant strains of M. tuberculosis. This initiative has been important to test first-line TB drugs and promoting research to develop new drugs, vaccines, and diagnostic strategies.1,4
Among the techniques used, whole genomic sequencing (WGS) is worth mentioning, especially for the investigation of bacilli resistance. This approach enables accurate assessment of mutations related to bacilli resistance to several TB drugs. This information would allow the development of new diagnostic methods and therapeutic strategies applied for disease control.5
Outhred et al. advocate the use of WGS for all multidrug-resistant M. tuberculosis isolates as an alternative plan to improve patient care, monitor for transmission events, and contribute to better understanding of resistance-associated mutations.6
This study describes a challenging case of treatment failure of a patient under MDR-TB therapy and reports phenotypic and molecular drug resistance test results in correlation to mutations identified with a whole genome sequencing analysis.
Three isolates of M. tuberculosis from a single patient, during different time points of his treatment, were evaluated based on phenotyping and genotyping testing: isolate 1 was collected before the patient started the follow-up in the reference center, when clinical and microbiological failure was diagnosed, despite regular TB treatment with rifampicin (R), isoniazid (H), pyrazinamide (Z) and ethambutol (E); isolate 2 was collected in the ninth month of MDR-TB treatment (other clinical and microbiological failure); and isolate 3 was obtained on the 13th month of MDR-TB treatment.
A nonradiometric phenotypic susceptibility testing was performed in liquid medium (MGIT 960; Becton Dickinson Diagnostic Systems, Sparks, MD) for isolate 1. Besides this susceptibility phenotypic testing, two line probe assays (LPA), Genotype MTBDRplus and MTBDRsl (Hain Lifescience, GmbH, Germany), were performed in isolates 2 and 3. Genotype MTBDRplus evaluates the main mutations associated with rifampicin (rpoB gene mutations) and isoniazid (katG e inhA mutations) resistance. Genotype MTBDRsl detects mutations related with resistance to fluoroquinolones (gyrA gene mutations), second-line injectable drugs (SLID) (rrs gene mutations) and ethambutol (embB gene mutations).7,8 Finally, the two stored samples (isolates 2 and 3) were submitted to WGS analysis using Illumina MiSeq Sequecing System (Illumina, San Diego, CA, USA). LPAs and WGS tests were performed with stored isolates 2 and 3 at the end of the patient's treatment. Therefore, this information was not available to the clinician during the treatment. Isolate 1 was not tested again because it was not viable.
Generated reads with phred scale score superior to 30 was mapped with BWA v0.7.5a program (Burrows-Wheeler Alignment Tool) using the reference genome M. tuberculosis H37Rv. Conversion from sequence alignment map format to sorted, indexed BAM files was done using SAMtools (version 0.1.19). PCR-duplicates were removed using the MarkDuplicates option of the Picard software tools (version 1.61). The variants were found according to the pipeline SAMtools/BCFtools v 0.1.18 and annotated with SnpEff v 4.0. Databases TB Drug Resistance Mutation Database9 and M. tb Drug Resistance Directed Sequencing Database10 were used to identify mutations described for the TB bacilli. All detected mutations were confirmed based on TB profiler online tool, described by Coll et al.,11 to remove single nucleotide polymorphisms (SNPs) at drug resistance loci which were historically misclassified as drug resistance markers.
This project was approved by the Ethics and Research Committee of the Hospital of the Ribeirão Preto Medical School of the University of São Paulo (protocol number: 944117 – February 1, 2015).
The patient was initially treated with RHZE for six months. During the last month clinical and microbiological failure (acid-fast smear positive and respiratory symptoms) was diagnosed. This M. tuberculosis isolate showed R and H resistance in the first phenotypic susceptibility testing. The patient was referred to the regional TB drug resistance center and his treatment was switched to streptomycin (Sm), E, ofloxacin (FQ), Z, and terizidone (Tz), following the Brazilian guidelines for the management of MDR-TB. During the first nine months of treatment, there was a transient improvement followed by recurrence of symptoms. Sputum culture was collected and the same MDR-TB treatment was maintained until the 13th month. The therapy was empirically optimized by adding ethionamide, extending the period of streptomycin and increasing the local support for directly observed treatment, while the results of phenotypic testing from isolate 3 were still pending. The treatment was successfully completed after 24 months, with clinical and microbiological cure. After that, instigated by this unusual and intriguing case and its outcome, we decided to carry on molecular studies on the patient's isolates 2 and 3.
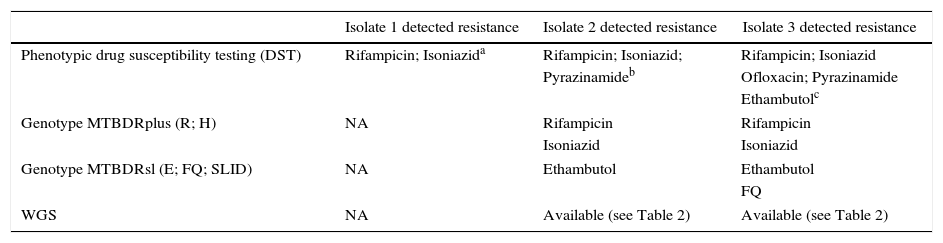
Isolate 1 showed resistance to R and H in the phenotypic test. Phenotypic testing of isolate 2 showed resistance to these two drugs and also to Z. The LPA of this isolate showed resistance to R (lost wild type 8 and gained rpoB S450L mutation), H (lost wild type and gained KatG S315T1 mutation), and E (lost wild type 1 and gained embB M306V mutation). Isolate 3 showed resistance to R, I, E (same mutations described in isolate 2), and acquired a new resistance pattern to FQ (gyrA D94G mutation) in the LPA test. Phenotypic testing of isolate 3, which became available close to the end of the patient's treatment, showed resistance to the above mentioned drugs and also to Z. The critical concentrations of Bactec-MGIT 960™ reported by the manufacturer's drug susceptibility testing (DST) protocol were as follows: H: 0.10μg/mL; R: 1.0μg/mL; EM: 5.0μg/mL; Sm: 1.0μg/mL; Z: 100μg/mL; FQ (ofloxacin): 2μg/mL; Amikacin 1.0μg/mL; Capreomycin 2.5μg/mL. The susceptibility profile of isolates in the phenotypic test and LPA are described in Table 1.
Results of phenotypic and LPA tests: susceptibility profile of tested drugs.
| Isolate 1 detected resistance | Isolate 2 detected resistance | Isolate 3 detected resistance | |
|---|---|---|---|
| Phenotypic drug susceptibility testing (DST) | Rifampicin; Isoniazida | Rifampicin; Isoniazid; | Rifampicin; Isoniazid |
| Pyrazinamideb | Ofloxacin; Pyrazinamide | ||
| Ethambutolc | |||
| Genotype MTBDRplus (R; H) | NA | Rifampicin | Rifampicin |
| Isoniazid | Isoniazid | ||
| Genotype MTBDRsl (E; FQ; SLID) | NA | Ethambutol | Ethambutol |
| FQ | |||
| WGS | NA | Available (see Table 2) | Available (see Table 2) |
NA, not available; R, rifampicin; H, isoniazid; E, ethambutol; FQ, fluoroquinolones; SLID, second line injectable drugs.
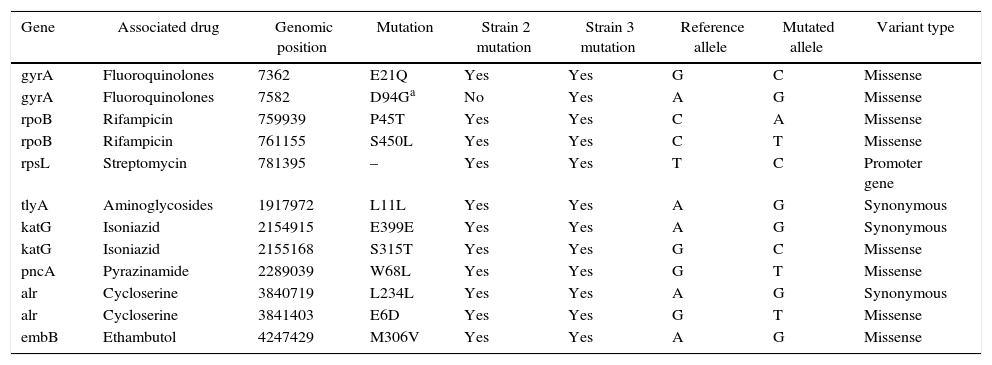
The WGS analysis of these two strains generated the total of 43,036,497 reads (28,171,267 for strain 1 and 14,865,230 for strain 2), with an average coverage of 469× for the isolate 2 and 244× for the isolate 3. Bioinformatics analysis reported 11 mutations already described as associated with resistance in isolate 2 and 12 mutations in isolate 3 (Table 2). Additional mutation in gyrA gene (D94G) was identified in isolate 3, which is one of the most frequent mutations associated with resistance to FQ, and is tested by the LPA Genotype MTBDRsl. Although all mutations showed by LPA were validated by WGS, additional mutations were detected, including those conferring resistance to other drugs despite bacilli susceptibility demonstrated in the phenotypic drug susceptibility testing (gyrA E21Q in isolate 2, rpsL promoter gene in genomic position 781395, tlyA L11L for isolates 2 and 3).
Whole genomic sequencing mutations identified in isolates 2 and 3.
| Gene | Associated drug | Genomic position | Mutation | Strain 2 mutation | Strain 3 mutation | Reference allele | Mutated allele | Variant type |
|---|---|---|---|---|---|---|---|---|
| gyrA | Fluoroquinolones | 7362 | E21Q | Yes | Yes | G | C | Missense |
| gyrA | Fluoroquinolones | 7582 | D94Ga | No | Yes | A | G | Missense |
| rpoB | Rifampicin | 759939 | P45T | Yes | Yes | C | A | Missense |
| rpoB | Rifampicin | 761155 | S450L | Yes | Yes | C | T | Missense |
| rpsL | Streptomycin | 781395 | – | Yes | Yes | T | C | Promoter gene |
| tlyA | Aminoglycosides | 1917972 | L11L | Yes | Yes | A | G | Synonymous |
| katG | Isoniazid | 2154915 | E399E | Yes | Yes | A | G | Synonymous |
| katG | Isoniazid | 2155168 | S315T | Yes | Yes | G | C | Missense |
| pncA | Pyrazinamide | 2289039 | W68L | Yes | Yes | G | T | Missense |
| alr | Cycloserine | 3840719 | L234L | Yes | Yes | A | G | Synonymous |
| alr | Cycloserine | 3841403 | E6D | Yes | Yes | G | T | Missense |
| embB | Ethambutol | 4247429 | M306V | Yes | Yes | A | G | Missense |
The relation between genome mutations and phenotypic resistance is particularly important for therapeutic decision, because different mutations can cause different resistance profiles or not even cause any phenotypic resistance.12 Coll et al. compiled a library of mutations predictive of drug resistance, and removed phylogenetic SNPs at drug resistance loci, which were historically misclassified as drug resistance markers.11
WGS has great application potential to detect bacilli resistance in clinical practice. However, further work is needed to determine additional resistance polymorphisms as it should be noted that high positive predictive values are crucial for drug resistance tests where the consequence of a false positive result may lead to unnecessary treatment and prolonged patient isolation.11
The WGS of M. tuberculosis is a great advance in the knowledge of bacilli resistance, as well as in the clinical management of TB. This technique presents greater discriminatory power, enabling analysis of additional mutations, not possible to be assessed by other methods. WGS has the potential for clinical use, as mentioned by other authors6,11–14 for fast and accurate assessments in cases of illness caused by strains resistant to multiple drugs, with an impact on therapeutic decisions. However, this is a high-cost technology and it is still critical to understand and standardize correlations between genotypic and phenotypic resistance to really optimize its clinical use.
Conflicts of interestThe authors declare no conflicts of interest.