Human T-cell lymphotropic virus type 1 (HTLV-1) is sexually transmitted and causes persistent infection. This virus induces activation of the immune system and production of inflammatory cytokines. This study aimed to assess the cytokine profile and cytopathological findings in the cervicovaginal fluid of asymptomatic HTLV-1-infected women.
MethodsHTLV-1-infected and uninfected women were selected at the Centro de Atendimento ao Portador de HTLV in Salvador-Brazil. None of the included HTLV-1-infected women reported any HTLV-1-associated diseases. All volunteers underwent gynecological examination to collect cervicovaginal fluid. Cytokine quantification was performed using the Cytometric Bead Array (CBA) Human Th1/Th2/Th17 kit. Light microscopy was used to evaluate cervicovaginal cytopathology. In addition, proviral load in cervicovaginal fluid and peripheral blood was measured by real-time quantitative polymerase chain reaction.
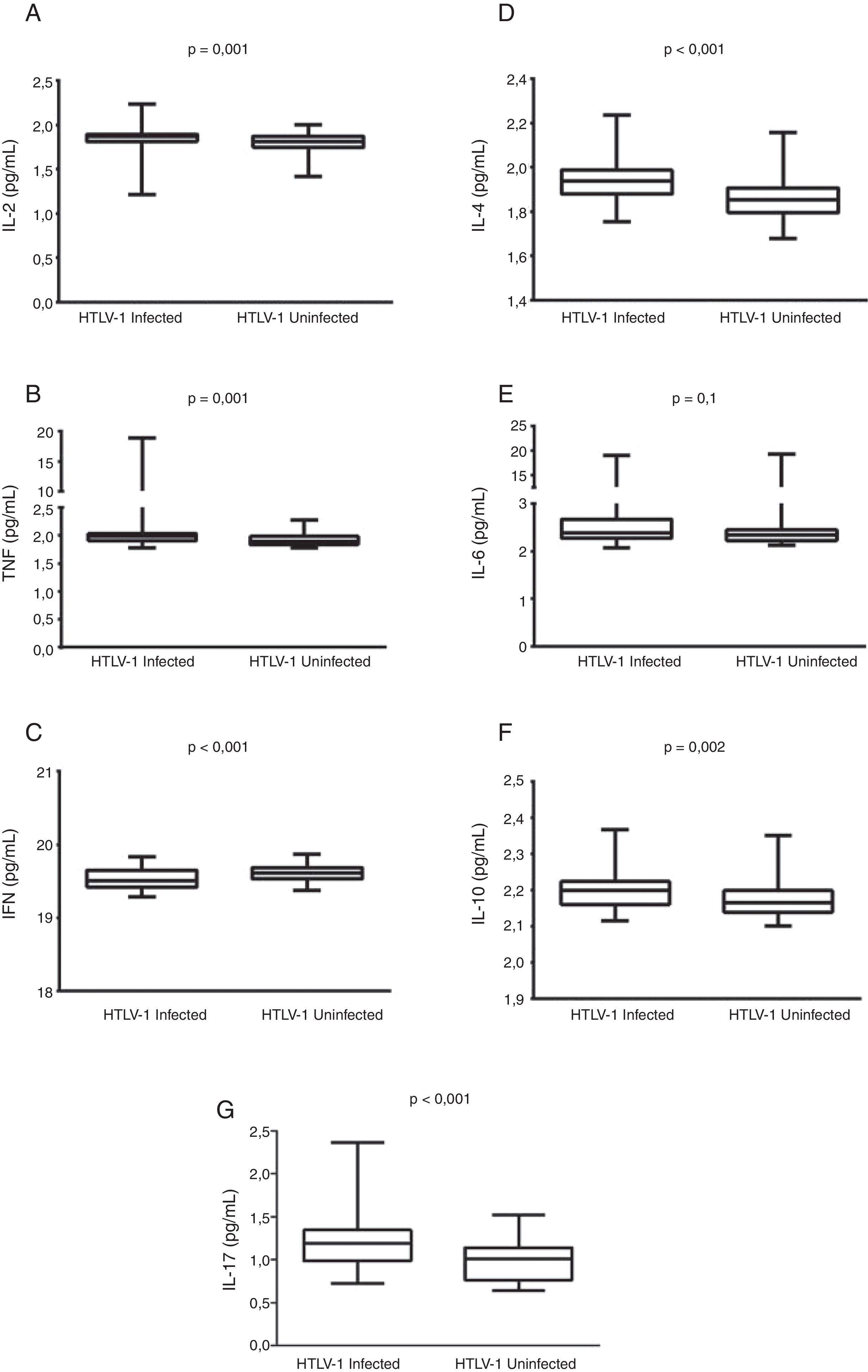
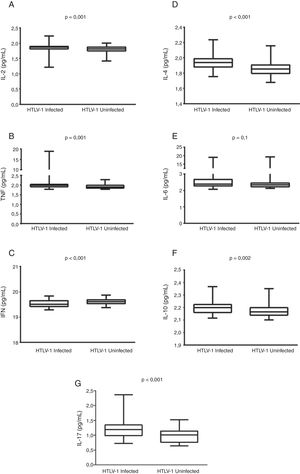
Results112 women (63 HTLV-1-infected and 49 uninfected) were evaluated. No differences were found with respect to cytopathological cervicovaginal findings between the groups. IL-2, TNF, IL-4, IL-10, and IL-17 levels were significantly higher in cervicovaginal fluid of the HTLV-1-infected women than in uninfected women (p<0.05). Conversely, IFN-γ was found to be lower in the HTLV-1-infected women (p<0.001) compared to uninfected individuals. Cervicovaginal proviral load was detectable in 53% of the HTLV-1-infected women and was found to be consistently lower than the proviral load in peripheral blood.
ConclusionsHTLV-1 infection induces immune activation in cervicovaginal environment, characterized by elevated concentrations of Th1, Th2, and IL17 in the cervicovaginal fluid.
Human T-cell lymphotropic virus type 1 (HTLV-1) is a retrovirus distributed worldwide, with endemic areas found in Africa, South and Central America, the Caribbean, Japan, Melanesia, and the Middle East. In Brazil, it is estimated that approximately 800,000 people are infected.1 This virus is transmitted vertically from mother to child, mainly by breastfeeding, or horizontally through transfusion of blood, contaminated needles, or sexual intercourse. A recent study conducted in Salvador, Brazil, underscored the importance of the sexual route in HTLV-1 transmission.2 In this city, HTLV-1-infection is more prevalent in women, reaching 10% of those over the age of 50.3
HTLV-1 is the etiological agent of adult T-cell leukemia/lymphoma (ATLL),4 HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP),5 HTLV-1 associated uveitis,6 and infective dermatitis in children.7 In addition, inflammatory diseases, such as keratoconjunctivitis sicca (KCS),8 bronchiectasis9 and arthritis10 are often associated with this viral infection.
Memory CD4+ T-lymphocytes are the main target cells for HTLV-1, although CD8+ T-lymphocytes, glial cells, and circulating dendritic cells may also represent targets for infection.11–13 Immune system activation is a hallmark of HTLV-1 infection, resulting in spontaneous lymphoproliferation, increased expression of activation markers (HLA-DR, CD25), and exacerbated production of proinflammatory cytokines (IFN-γ, TNF) and chemokines (IL-8, CXCL9 and CXCL10).14,15 Studies indicate that elevated proviral load (>5% of infected cells) and immune activation are commonly found in the context of HTLV-1-associated diseases, as compared to asymptomatic HTLV-1 carriers.16–20 However, asymptomatic individuals with low proviral load may also present pronounced immune activation.21
Few studies have evaluated the effect of HTLV-1 infection in the vaginal mucosa. Detection of HTLV-1 DNA in the cervical fluid of infected women was previously associated with cervicitis.22 In addition, presence of anti-HTLV-1 in the vaginal fluid has been described, including in asymptomatic HTLV-1 infected women.23 This study evaluates the cervicovaginal environment of HTLV-1 infected women by assessing proviral load, cytopathological alterations, and cytokine levels.
MethodsRecruitment and study designThe present cross-sectional observational study was conducted at the Centro de Atendimento ao Portador de HTLV of the Escola Bahiana de Medicina e Saúde Pública, Salvador, Bahia, Brazil. Asymptomatic HTLV-1 infected women were sequentially included in the course of routine medical consultations from August 2014 to March 2016. All individuals were included if their neurologic examination was normal and no complaints for HTLV-1-associated diseases were reported. Uninfected women (controls) were selected from companions or relatives of patients who attended consultations. These individuals were paired to HTLV-1-infected women matched for age, presence of comorbidities, smoking, contraceptive methods, and presence of other sexually transmitted infections. The sample size for the HTLV-1 asymptomatic group was calculated based on a 30% estimated prevalence of sexual dysfunction for HTLV-1 uninfected women, with an estimated prevalence ratio (PR) of 2.0 among both the HTLV-1-infected and uninfected women. Adopting an alpha error of 5% and power of 80%, the necessary sample size was determined to be 49 women in each group. The inclusion criteria consisted of age ranging from 20 to 50 years and report of sexual activity within four weeks preceding the consultation. Women with HTLV-1-associated diseases, those who were menopausal or diagnosed with depression, as well as those taking medication known to affect sexual desire (beta blockers, antidepressants, central nervous system depressants or anticholinergics) were excluded. HTLV-1 infection was diagnosed by Enzyme-Linked Immunosorbent Assay (ELISA) with Western Blot used for confirmation. This study was approved by the Institutional Research Board of the Escola Bahiana de Medicina e Saúde Pública (registered under protocol: CAAE 33098414.4.0000.5544) and all included women signed a term of informed consent.
Collection and analysis of samplesFollowing the routine medical consultation, demographic, medical, sexual, and gynecological data were obtained through specific standardized data collection forms, and physical and gynecological examinations were performed. Papanicolaou smears were collected from the ectocervix and endocervix using an Ayres spatula and cytobrush, respectively. Cotton swabs were used to collect vaginal fluid from the ectocervix, endocervix and vaginal walls for proviral load (PVL) measurement and cytokine quantification.
Cell abnormalities detected in the Papanicolaou smears were classified in accordance with the Bethesda System.24 To measure PVL in the cervicovaginal fluid, swabs were placed in tubes containing 400μL of hydroxymethyl-ethylene diamine tetra acetic acid (Tris-EDTA) solution and stored at −20°C until use. For cytokine quantification, swabs were preserved in cryotubes containing 1mL of sterile phosphate-buffered saline (PBS) stored at −70°C. Cytokine levels were assessed by flow cytometry using the Cytometric Bead Array (CBA) Human Th1/Th2/Th17 kit (Becton, Dickinson and Company, New Jersey, USA). Whole blood samples were collected in EDTA tubes and peripheral blood mononuclear cells (PBMC) were obtained by density gradient centrifugation and cryopreserved until use. HTLV-1 PVL in cervicovaginal cells and PBMCs was determined by real-time TaqMan PCR, as described elsewhere.25
Statistical analysesData were expressed as medians and percentiles (25th and 75th) or means and standard deviation. Differences between HTLV-1 infected and uninfected women concerning family income, schooling, length of relationship, number of partners, parity, proviral load, and cytokine levels were assessed by the non-parametric Mann–Whitney U test, and differences in age were evaluated by the Student's t-test. Differences in qualitative variables (skin color, marital status and cytopathological/vaginal microbiota) were assessed using the Chi-square test. Spearman's rank correlation coefficient was used to determine associations between cytokine levels and proviral load. p-values less than 0.05 were considered statistically significant. All analyses were performed using GraphPad software version 5.0 and SPSS software version 17.0 for Windows.
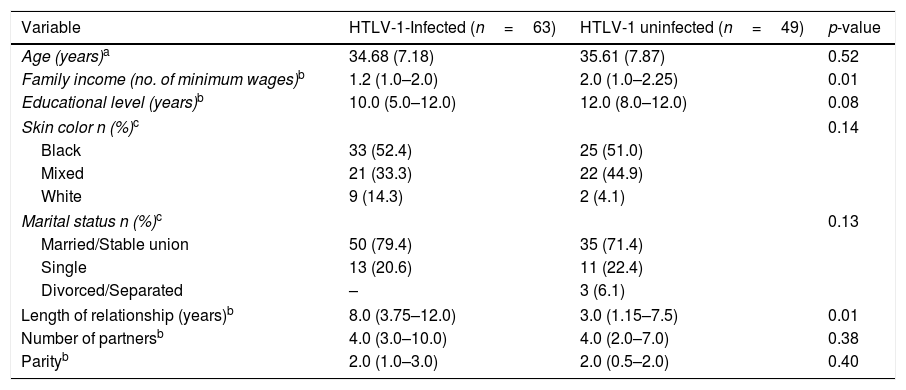
ResultsA total of 112 women (63 infected with HTLV-1 and 49 uninfected) were evaluated (Table 1). No significant differences between the groups were seen regarding sociodemographic profile, number of partners, or parity. HTLV-1-infected women had longer relationships than uninfected women (p=0.01) and the median income of HTLV-1-infected women (1.2 minimum wage) was lower compared to that of uninfected women (2 minimum wages) (p=0.01). HTLV-1 proviral load was detected in 94% of the PBMC samples from the infected women, with a median of 28,665copies/106 cells (IQR 4868–69,408copies/106 cells).
Sociodemographic profile of HTLV-1-infected and uninfected women.
| Variable | HTLV-1-Infected (n=63) | HTLV-1 uninfected (n=49) | p-value |
|---|---|---|---|
| Age (years)a | 34.68 (7.18) | 35.61 (7.87) | 0.52 |
| Family income (no. of minimum wages)b | 1.2 (1.0–2.0) | 2.0 (1.0–2.25) | 0.01 |
| Educational level (years)b | 10.0 (5.0–12.0) | 12.0 (8.0–12.0) | 0.08 |
| Skin color n (%)c | 0.14 | ||
| Black | 33 (52.4) | 25 (51.0) | |
| Mixed | 21 (33.3) | 22 (44.9) | |
| White | 9 (14.3) | 2 (4.1) | |
| Marital status n (%)c | 0.13 | ||
| Married/Stable union | 50 (79.4) | 35 (71.4) | |
| Single | 13 (20.6) | 11 (22.4) | |
| Divorced/Separated | – | 3 (6.1) | |
| Length of relationship (years)b | 8.0 (3.75–12.0) | 3.0 (1.15–7.5) | 0.01 |
| Number of partnersb | 4.0 (3.0–10.0) | 4.0 (2.0–7.0) | 0.38 |
| Parityb | 2.0 (1.0–3.0) | 2.0 (0.5–2.0) | 0.40 |
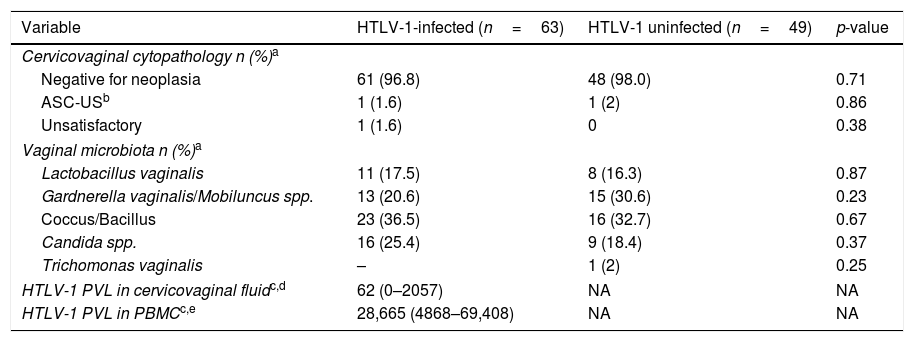
Regarding cytopathological findings were similar among HTLV-1 infected or uninfected women with negative results for neoplasia (p=0.71) (Table 2). Atypical squamous cells of undetermined significance (ASC-US) was identified in 1.6% and 2% of infected and uninfected women, respectively (p=0.86). In addition, similar vaginal microbiota (Lactobacillus vaginalis, Gardnerella vaginalis/Mobiluncus spp., Coccus/Bacillus, Candida spp., and Trichomonas vaginalis) were found in the two groups. HTLV-1 proviral load was detectable in 53% of the cervicovaginal samples from HTLV-1-infected women. The median cervicovaginal proviral load [62copies/106 cells (IQR 0–2057copies/106cells)] was consistently lower than that in peripheral blood [28,665copies/106 cells (IQR 4868–69,408)].
Frequency of cervicovaginal cytopathologic findings and HTLV-1 proviral load in cervicovaginal and PBMC samples.
| Variable | HTLV-1-infected (n=63) | HTLV-1 uninfected (n=49) | p-value |
|---|---|---|---|
| Cervicovaginal cytopathology n (%)a | |||
| Negative for neoplasia | 61 (96.8) | 48 (98.0) | 0.71 |
| ASC-USb | 1 (1.6) | 1 (2) | 0.86 |
| Unsatisfactory | 1 (1.6) | 0 | 0.38 |
| Vaginal microbiota n (%)a | |||
| Lactobacillus vaginalis | 11 (17.5) | 8 (16.3) | 0.87 |
| Gardnerella vaginalis/Mobiluncus spp. | 13 (20.6) | 15 (30.6) | 0.23 |
| Coccus/Bacillus | 23 (36.5) | 16 (32.7) | 0.67 |
| Candida spp. | 16 (25.4) | 9 (18.4) | 0.37 |
| Trichomonas vaginalis | – | 1 (2) | 0.25 |
| HTLV-1 PVL in cervicovaginal fluidc,d | 62 (0–2057) | NA | NA |
| HTLV-1 PVL in PBMCc,e | 28,665 (4868–69,408) | NA | NA |
HTLV-1-infected women had significantly higher concentrations of IL-2, TNF, IL-10, IL-4, and IL-17 in the cervicovaginal fluid than uninfected women (p<0.05). Conversely, these women presented lower concentrations of IFN-γ in the vaginal fluid compared to those uninfected by HTLV-1 (p<0.001) (Fig. 1). The IFN-γ/IL-10 ratio in HTLV-1-infected women (8.87, IQR 8.77–9.10) was significantly lower than in uninfected women (9.05, IQR 8.88–9.23), (p<0.001). Cytokine levels were not found to be correlated with HTLV-1 proviral load in either the cervicovaginal fluid or in peripheral blood mononuclear cells.
DiscussionThe present results indicate that, in addition to elevated levels of regulatory IL-10, both Th1 (IL-2 and TNF) and Th2 (IL-4) cytokines, as well as IL-17, were all higher in the cervicovaginal fluid of HTLV-1-infected women as compared to uninfected individuals. This immune activation of vaginal environment may be consequent to the presence of infected cells in the vaginal mucosa. Indeed, HTLV-1 proviral load was found to be detectable in the vaginal fluid of more than 50% of the infected women. Corroborating our results, Belec et al. found viral DNA in three out of 15 HTLV-1-infected women and suggested that the virus induced a local immune response that resulted in increased levels of HTLV-1 antibodies in the vaginal fluid.23 In addition, the presence of HTLV DNA in cervical samples obtained from HTLV-1-infected sex workers was associated with the diagnosis of cervicitis.22
It has been well established that HTLV-1 induces activation of the immune system, which is reflected by spontaneous proliferation of peripheral blood mononuclear cells and production of cytokines. Individuals with a diagnosis of HAM/TSP commonly present higher plasma levels of proinflammatory cytokines (e.g. IFN-γ, TNF, IL-6) and chemokines (CXCL9, CXCL10) than asymptomatic or uninfected individuals.14,16,26 Moreover, other HTLV-1-associated conditions, such as neurogenic bladder,27 infective dermatitis17 and sicca syndrome,18 have also been reported in association with higher plasma levels of IFN-γ and IL-6.16 In addition, high production of IFN-γ is found in asymptomatic HTLV-1-infected individuals compared to uninfected controls.14 In the present study, low levels of IFN-γ and lower IFN-γ/IL-10 ratios were found in the cervicovaginal samples from the HTLV-1-infected group. The regulatory cytokine IL-10 has been reported to have an antagonist effect on the production of IFN-γ.28 The predominance of IL-10 and TGF-β is commonly found in the mucosa of healthy individuals, which creates a regulatory milieu that maintains an immunological tolerance against antigens from microbiota and the external environment.29,30 The significantly higher IL-10 levels found in the cervicovaginal fluid of HTLV-1-infected women compared to uninfected women seems to suggest that the presence of the virus induced increased immune regulation.
Similarly, women infected with HIV, another retrovirus, also present increased levels of Th1 and Th2 cytokines in the vaginal fluid as compared to uninfected controls.30,31 Regarding IL-17, a study conducted in HIV-1-infected women found higher IL-17 concentrations in the vaginal fluid of women with sexually transmitted bacterial infections than in those without these types of infections, whereas women with Candida spp. had lower IL-17 concentrations compared to those without candidal infections.32
It has been demonstrated that local immune activation induced by HIV in the vaginal environment may modulate virus shedding in cervicovaginal secretions.33 It is possible that HTLV-1 induces a similar immune activation, thereby increasing cytokine production in situ. On the other hand, it is theoretically possible that elevated levels of cytokines detected in vaginal fluid may also be the result of another phenomenon, such as vaginal transudation, a natural process that allows vaginal lubrication through vaso-dilatation.34
A positive correlation between inflammatory cytokine levels and proviral load in the blood was described in HTLV-1-infected individuals diagnosed with Sicca syndrome.18 However, our study found no correlations between the levels of cytokines in vaginal fluid and proviral load in either cervicovaginal fluid or PBMCs. Of note, the HTLV-1-infected women evaluated herein were asymptomatic for diseases associated with this virus and had very low PVL in the vaginal fluid, 62copies/106 cells, about 0.006% of infected cells, in addition to intermediate levels of proviral load in the blood (28,665copies/106 cells, about 2.9% of infected PBMCs). For comparison, HTLV-1 PVL in peripheral blood is considered low when <1% of PBMCs are infected and intermediate only when 1–5% of PBMCs are infected.20,35
With respect to the cytopathological findings and vaginal microbiota, no differences were observed between HTLV-1-infected and uninfected women. Higher levels of Th1 and Th2 cytokines were found in women with cervical intraepithelial neoplasia, with increasing levels seen in accordance with lesion severity.29,36 In our study, one woman from each group had atypical squamous cells of undetermined significance, a cytopathological alteration that requires periodic monitoring.
A limitation of the present study was the absence of HTLV-1-infected women diagnosed with HAM/TSP, who consistently present higher proviral loads in the peripheral blood as compared to asymptomatic individuals. However, HTLV-1 proviral load in the cervicovaginal fluid was indeed detectable in the majority of HTLV-1-asymptomatic women herein. Another limitation was that as cytokine levels in the plasma were not quantified, it was impossible to pair these with those in the cervical fluid.
ConclusionsThe results presented herein show that HTLV-1 infection induces immune activation in the cervicovaginal environment of asymptomatic women, characterized by elevated levels of Th1, Th2, and IL17 cytokines in the cervicovaginal fluid. Further studies should be conducted involving HTLV-1-infected women who present high levels of proviral load in the peripheral blood to determine whether correlations exist regarding proviral load in cervicovaginal fluid.
Financial supportThis work was supported by the Fundação de Amparo à Pesquisa do Estado da Bahia (BOL0575/2015). This study was financed in part by the Coordenaçãao de Aperfeiçãoamento de Pessoal de Nível Superior (CAPES) - Finance Code 001
Conflicts of interestThe authors declare no conflicts of interest.
We thank Mr. Noilson Lazaro and Viviana Olavarria for technical assistance. The authors would like to thank Andris K. Walter for English revision/editing services.