A prospective study was conducted in four tertiary hospitals in Argentina and Mexico in order to describe the occurrence of Clostridium difficile infection (CDI) in these settings. The objective was to evaluate the incidence of CDI in at-risk populations in Argentina (one center) and Mexico (three centers) and to further explore potential study sites for vaccine development in this region. A prospective, descriptive, CDI surveillance study was conducted among hospitalized patients aged ≥40 years who had received ≥48h of antibiotic treatment. Stool samples were collected from those with diarrhea within 30 days after starting antibiotics and analyzed for toxins A and B by ELISA, and positive samples were further tested by toxinogenic culture and restriction endonuclease analysis type assay. Overall, 466 patients were enrolled (193 in Argentina and 273 in Mexico) of whom 414 completed the follow-up. Of these, 15/414 (3.6%) experienced CDI episodes occurring on average 18.1 days after admission to hospital and 15.9 days after the end of antibiotics treatment. The incidence rate of CDI was 3.1 (95% CI 1.7–5.2) per 1000 patient-days during hospitalization, and 1.1 (95% CI 0.6–1.8) per 1000 patient-days during the 30-day follow-up period. This study highlighted the need for further evaluation of the burden of CDI in both countries, including the cases occurring after discharge from hospital.
Clostridium difficile (CD) is a potentially deadly,1–5 spore-forming bacterium1 that is emerging as a leading cause of life-threatening, healthcare-acquired infections worldwide.3–11 Pathogenic strains of CD produce potent toxins (e.g. toxA, toxB) that cause the clinical manifestations in humans known as symptomatic CD infection (CDI),4,8,12,13 especially in vulnerable individuals and mostly in hospitals and long-term care facilities.1,2,5,7,13–18 An increased prevalence of CDI associated with an increase in disease severity and mortality has been documented in the United States, Canada and Europe.
Individuals most at-risk for CDI have a generally weakened condition,4,7,19,20 have received antibiotics,1,4,7,9,12,15,17,18,21,22 and are likely to have been hospitalized or residing in long-term care facilities,1,2,7,12,13,15,17,18,22 where they could have been exposed to environmental spores. Also, age correlates with increased incidence, severity of disease, the likelihood of recurrences, and CDI related-death.2–4,7,9,12,13,21–25Despite the availability of antibiotics to treat CDI, there is a disturbingly large proportion of patients (i.e. 20–30%) who experience recurrences of CDI,9,12,13,16,19,20,25 which lead to re-hospitalizations20 and longer hospital stays.4,18,20,25 Since transmission of CD is difficult to control14 and treatment options for CDI are less than ideal,4,13,19,20 vaccination could be an efficacious,13,16 cost-effective1,4,17,19,20,24–26 and complementary public health measure12,13 to protect vulnerable individuals from this devastating disease, in whom the attributable mortality is 8–15%.3,5 Sanofi Pasteur is developing a toxoid vaccine for the prevention of primary CDI in at-risk individuals. In addition, a number of other groups, both public agencies and private corporations, are developing and evaluating a number of other prophylactic approaches and management strategies.
Data on the frequency and impact of CDI in Latin America are sparse6,27–33; however the disease has been reported from prospective laboratory surveillance and in outbreaks.6 Moreover, more virulent CD ribotype 027 has been isolated in Costa Rica32 and in Chile.34 We therefore conducted a prospective epidemiological study in order to estimate the incidence of symptomatic laboratory-confirmed CDI cases in Argentina and Mexico among newly hospitalized patients considered at risk (≥40 years of age who have commenced in-hospital antibiotic treatment) and to describe the characteristics of these patients, as a prelude for potential vaccine trials in this region.
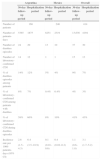
Materials and methodsType of study and settingThis study was a prospective, descriptive, surveillance of CDI in hospitalized patients conducted in one hospital in Argentina and in three hospitals in Mexico, from September 21, 2010 to September 19, 2011. Study sites are described in Table 1.
Description of the institutions involved in the study.
| Argentina | Mexico | |||
|---|---|---|---|---|
| Place | Buenos Aires, municipality of Vincente López | Toluca | Guadalajara | Veracruz |
| Name | Hospital Municipal Prof. Dr. Bernardo Houssay, de Vicente López | Centro Médico “Lic. Adolfo López Mateos” | Antiguo Hospital Civil de Guadalajara “Fray Antonio Alcalde” | Hospital de Alta Especialidad de Veracruz “Virgilio Uribe” |
| Size of recruitment area | 275,000 | 2 million | 1.6 million | 512,000 |
| Type of institution | Tertiary care, teaching | Tertiary care | Tertiary care, research teaching | Tertiary care |
| Number of specialties | 25 | 31 | 35 | 18 |
| Beds | 142 | 200 | 733 | 244 |
| Population | Adults, children | Adults | Adults, children | Adults, children |
Patients aged ≥40 years and who received at least 48h of antibiotic treatment were included in this study. Excluded patients were those with CDI within the last three months, or who presented with diarrhea at admission or who received antibiotics within 30 days prior to admission or started on antibiotic treatment beyond 48h of hospitalization.
Case definitionA laboratory-confirmed CDI case was defined as a patient with clinical suspicion of CDI (i.e. antibiotic-associated diarrhea) and ELISA test detection of CD toxins A or B. Diarrhea was defined as three or more stools in a 24-h period. A severe CDI case was defined as a laboratory-confirmed CDI case with at least two of the following criteria: white blood cell (WBC) count >15,000/mL, >50% increase on serum creatinine, temperature >38.5°C, evidence of severe colitis (i.e. abdominal or radiological signs), age at the time of CDI ≥60 years, or hospitalized in intensive care unit (ICU) during CDI.
Laboratory testingStool samples were collected from patients with diarrhea within 30 days after the start of antibiotic therapy and underwent confirmatory laboratory testing using ELISA for C. difficile toxins A and B, and toxigenic culture with restriction endonuclease analysis (REA) typing on ELISA-positive stools. Briefly, stool mixed with phosphate buffered saline (PBS) at a dilution of 1:40 was centrifuged, and the supernatant filtered on a 0.45μm pore-size filter, and inoculated on Hep-2 cell monolayers. Toxigenic culture samples were selected to perform a neutralization assay with Clostridium sordelli anti-toxin.
Data collectionEach included patient was followed up 30 days after the start of antibiotic therapy. The following data were collected for each recruited subject: dates of admission and discharge, demographics, medical history including hospitalizations and surgeries, reasons for admission to the hospital, prior history of CDI, history of co-morbidities, and history of antibiotics use, as well as use of antibiotics, proton pump inhibitors and/or probiotics throughout the 30-day follow-up period. If applicable, onset of the first CDI episode, and antibiotic treatment for the CDI episode were also reported.
If a study participant was discharged from hospital before the completion of the 30-day follow-up period, he/she was given the material needed for stool sample, instructions on collection, and instructions to immediately contact the site in case of diarrhea. In addition, study staff called the patient's home every three to four days up to the end of the follow-up period to ask about any occurrence of diarrhea and to reinforce the need to report any such occurrences immediately. If diarrhea occurred, the subject was asked to bring the sample to the study site.
Statistical analysisData were described using absolute and relative frequencies. Incidence density rates of confirmed cases were calculated as the number of CDI cases per 1000 patient days, with their 95% confidence interval (CI). These rates were calculated in two ways, using either the study follow-up period or the hospital length of stay as the risk periods of developing CDI.
Quantitative variables were compared between groups using the Mann–Whitney U-test and categorical variables using the Fisher exact test. Statistical analyses were performed using SAS® 9.2 software, and p-value <0.05 were considered as statistically significant.
Ethics statementProtocol and informed consent forms (ICF) were approved by each institutional ethic committee before study initiation and ICF was obtained before inclusion.
ResultsA total of 466 patients were enrolled in the study and 414 (89%) completed the follow-up. Among the 52 (11%) patients who discontinued the study, the most common reasons for discontinuation were death during hospitalization (n=32; 7%) and lost to follow-up (n=12; 3%).
Demographics and hospitalization dataThe overall mean [range] age of included patients was 61.4 years [40; 95], 229 (55%) were male and 185 (45%) were female.
The most frequent reasons for hospitalization during the study were abdominal pain (n=28; 7%), pneumonia (n=33; 8%), cellulitis (n=14; 3%), dyspnea (n=14; 3%), pyrexia (n=13; 3%), and pain in extremity (n=13; 3%). The mean [range] length of stay was 9.0 days [2; 36 days] and at least one procedure or surgery was planned for 361 (87%) patients during hospitalization.
Description of patient historyNo patients had prior history of CDI. Twenty-eight (7%) patients were hospitalized in the last three months and only six (1%) patients were previously hospitalized or were in a long-term care facility within the 30-day period before study inclusion.
The majority (n=279; 67%) of the patients reported taking medication on a daily basis prior to their admission. Medications included proton-pump inhibitors (n=52; 13%), H2 receptor antagonists (n=26; 6%), immunosuppressive agents (n=5; 1%), and antibiotics within the last three months (but excluding the past 30 days) (n=25; 6%).
The most commonly prescribed antibiotics during hospitalization were third generation cephalosporins for 201 (49%) patients, fluoroquinolones for 153 (37%) patients, lincosamides for 98 (24%) patients and first generation cephalosporins for 96 (23%) patients.
Comparison of medication usage is presented in Table 2. No difference was observed between CDI positive and CDI negative patients, except for the following antibiotics which were significantly more frequently used among laboratory-confirmed cases than among CDI negative patients: clindamycin, combinations of penicillins (including β-lactamase inhibitors), vancomycin, carbapenems, trimethoprim and derivatives, sulfonamides, polymyxins, and penicillins with extended spectrum.
Patient characteristics of laboratory-confirmed and non-laboratory-confirmed Clostridium difficile infection (CDI) cases.
| Patient characteristics | Laboratory-confirmed CDI casesn=15 (%) | Non-laboratory-confirmed CDI casesn=399 (%) | p-value | Totaln=414 (%) |
|---|---|---|---|---|
| Age (years) | ||||
| Mean (min–max) | 67 (41–92) | 61 (40–95) | 0.056 | 61 (40–95) |
| Sex | ||||
| Male | 8 (53) | 221 (55) | 0.539 | 229 (55) |
| Female | 7 (47) | 178 (45) | 185 (45) | |
| Hospitalization history in the last 3 months | ||||
| No | 13 (87) | 373 (94) | 0.269 | 386 (93) |
| Yes | 2 (13) | 26 (7) | 28 (7) | |
| Proton pump inhibitors | ||||
| No | 12 (80) | 349 (88) | 0.417 | 361 (87) |
| Yes | 3 (20) | 49 (12) | 52 (13) | |
| Unknown | 0 | 1 | 1 | |
| Anti-H2 | ||||
| No | 15 (100) | 372 (93) | 0.613 | 387 (94) |
| Yes | 0 (0) | 26 (7) | 26 (6) | |
| Unknown | 0 | 1 | 1 | |
| Immunosuppressors | ||||
| No | 15 (100) | 393 (99) | >0.999 | 408 (99) |
| Yes | 0 (0) | 5 (1) | 5 (1) | |
| Unknown | 0 | 1 | 1 | |
| Antibiotics received in the last 3 months | ||||
| No | 13 (87) | 374 (94) | 0.229 | 387 (94) |
| Yes | 2 (13) | 23 (6) | 25 (6) | |
| Unknown | 0 | 2 | 2 | |
| Antibiotics received prior to onset of CDI | ||||
| Clindamycin | 11 (73) | 98 (25) | <0.001 | 98 (24) |
| 3rd-generation cephalosporins | 9 (60) | 192 (48) | 0.436 | 201 (49) |
| Combinations of penicillins, including β-lactamase inhibitors | 8 (53) | 88 (22) | 0.001 | 96 (23) |
| Fluoroquinolones | 7 (47) | 146 (37) | 0.427 | 153 (37) |
| Vancomycin | 5 (33) | 16 (4) | <0.001 | 21 (5) |
| Carbapenems | 4 (27) | 21 (5) | 0.009 | 25 (6) |
| Trimethoprim and derivatives | 3 (20) | 6 (2) | 0.003 | 9 (2) |
| Sulfonamides | 3 (20) | 6 (2) | 0.003 | 9 (2) |
| Nitroimidazole derivatives | 4 (27) | 72 (18) | 0.493 | 76 (18) |
| Macrolides | 2 (13) | 41 (10) | 0.662 | 43 (10) |
| 4th-generation cephalosporins | 1 (7) | 9 (2) | 0.312 | 10 (2) |
| Triazole derivatives | 1 (7) | 4 (1) | 0.169 | 5 (1) |
| Polymyxins | 1 (7) | 0 (0) | 0.036 | 1 (0) |
| Penicillins with extended spectrum | 1 (7) | 1 (0) | 0.071 | 2 (0) |
| Unspecified antibiotics | 1 (7) | 59 (15) | 0.707 | 60 (14) |
| Aminoglycosides | 1 (7) | 3 (1) | 0.138 | 4 (1) |
Table 3 reports estimates of diarrhea and laboratory-confirmed CDI by country and overall. Thirty-seven (9%) patients experienced at least one episode of diarrhea, occurring in hospital for 30 (81%) patients and at home for eight (22%) patients (one patient had two episodes, one in hospital and one at home). A total of 15 patients had laboratory-confirmed CDI, 13 occurring during the stay in hospital and two at home; 14 (8%) CDI were diagnosed in Argentina (including five severe cases) and one (0.4%) in Mexico (Veracruz). Overall, CD was found in 41% (15/37) of diarrhea cases and in 3.6% (15/414) of patients, but these estimates were higher in Argentina than in Mexico. The overall incidence density was 1.1 (95% CI 0.6–1.8) per 1000 patient-days during the study follow-up period and 3.1 (95% CI 1.8–5.0) per 1000 patient-days during hospitalization. Again, these figures were higher in Argentina than in Mexico.
Estimates of diarrhea and laboratory-confirmed Clostridium difficile infection (CDI).
| Argentina | Mexico | Overall | ||||
|---|---|---|---|---|---|---|
| 30-day follow-up period | Hospitalization period | 30-day follow-up period | Hospitalization period | 30-day follow-up period | Hospitalization period | |
| Number of patients | 168 | 246 | 414 | |||
| Number of patients-days | 5385 | 1875 | 8251 | 2319 | 13,636 | 4194 |
| Number of diarrhea episodes | 24 | 20 | 13 | 10 | 37 | 30 |
| Number of laboratory-confirmed CDI | 14 | 12 | 1 | 1 | 15 | 13 |
| % of diarrhea episodes among patients | 14% | 12% | 5% | 4% | 9% | 7% |
| % of laboratory-confirmed CDI among patients with diarrhea | 8% | 7% | 0.4% | 0.4% | 4% | 3% |
| % of laboratory-confirmed CDI during diarrhea episodes | 58% | 60% | 8% | 10% | 41% | 43% |
| Incidence rate per 1000 patients-days (95% CI) | 2.6 | 6.4 | 0.1 | 0.4 | 1.1 | 3.1 |
| (1.5–4.3) | (3.5–10.9) | (0.01–0.6) | (0.02–0.2) | (0.6–1.8) | (1.7–5.2) | |
Positive toxigenic culture was reported for 14 (93%) of the 15 laboratory-confirmed CDI, concerning 13 patients in Argentina and one patient in Mexico. Among the 13 Argentinean patients, 11 (85%) patients were infected with C. difficile REA type CF group and two (15%) patients were infected with DH group and Y group, respectively. A non-specific REA type was identified in the Mexican patient.
The median [range] time interval between admission and the first CDI episode was 18.1 days [3; 30] and the median [range] duration of antibiotics between onset of antibiotic therapy and the first CDI episode was 15.9 days [1; 28].
According to the definition, five (33%) severe CDI were observed among the 15 laboratory-confirmed CDI patients. They had an average of five diarrhea within 24h [3; 8] and reported clinical symptoms included abdominal pain (n=3), malaise (n=3), watery diarrhea (n=3), abdominal distention (n=2), fever ≥38°C (n=2), loss of appetite (n=2), weight loss (n=2) and hypotension (n=1).
DiscussionThis prospective multicenter study confirmed the presence of CDI in at-risk patients hospitalized in tertiary centers in Argentina and Mexico, with an incidence of 1.1 per 1000 patient days within 30 days after initiation of antibiotic treatment. CDI episodes also occurred after discharge from hospital, suggesting that follow-up of at-risk patients is essential to estimate the rate of CDI. In addition, the REA CF group observed predominantly in this study is also known as ribotype 017 and toxinotype VIII and was previously reported in outbreaks worldwide.15
Data on the frequency and impact of CDI in Latin America are sparse and comparison between studies is difficult due to the variety of study designs. One study conducted among 113 patients in Mexico and Latin America from 2003 to 200727 identified use of H2 blockers, prior hospitalization within 12 weeks of diagnosis, prior use of cephalosporins and fluoroquinolones, stay at an ICU, extended hospital stay, and antimicrobial use before diagnosis as risk factors for CDI. In our study, neither cephalosporins nor fluoroquinolones were found associated with CDI. From 1998 to November 1999, 245 fecal specimens from hospitalized and ambulatory patients were tested to confirm the diagnosis of CDI in a medical center in Buenos Aires (28, 29) and 16 (6.5%) were identified as positive by isolation of cytotoxigenic CD among hospitalized patients (81.3%) and outpatients (18.7%) with a mean age of 72.9 years. All patients had received two or more antimicrobial agents, mainly beta-lactams, two months before the appearance of diarrhea. In Argentina, 104 consecutive stool samples from 87 patients with diarrhea were screened for toxigenic CD between April 2000 and April 2001.31,33 The study reported that 38.5% of the samples and 36.8% of the patients were positive for CD. Another study performed in a 200-bed general hospital in Buenos Aires35 showed incidence rates of CDI ranging from 37 to 84 per 10,000 admissions between years 2000 and 2005. Finally, Quesada-Gomez and co-workers32 reported the emergence of CD NAP1 strain in a Costa Rican hospital.
Some limits and strengths of this descriptive study should be discussed. Data were collected in two geographical areas and four centers, which may limit the generalization of the risk estimates to Latin America. Outbreaks have been previously reported in Brazil and Argentina6 and ribotype 027 was already isolated in Costa Rica32 and in Chile.34 This suggested that CDI could tend to occur in outbreaks in Argentina, and this may explain why more cases were observed in this country than in Mexico after only one year of surveillance. Therefore, a longer surveillance period over a larger number of study sites and countries may be needed in order to have a more generalizable estimation of CDI in the region. In our study, results were consolidated by the prospective nature of the study design with laboratory confirmation of CDI using two complementary laboratory techniques (ELISA and REA). While polymerase chain reaction (PCR)-based methods may have allowed for an increased detection rate of toxigenic C. difficile than ELISAs for Toxin A and B, such methods were not in routine practice at the sites at the time of this study. In addition, these methods may fail to discriminate between CDI and asymptomatic colonization with C. difficile, potentially leading to an over detection of cases whereas ELISA-based toxin testing offers strong evidence of clinical disease despite its shortcomings with regards to sensitivity.36
While antibiotic therapy using fidaxomicin (approved in 2011 and not available at the time of this study), metronidazole, vancomycin, and rifampicin is often used in treating the principal episodes of diarrhea, these agents are of no benefit in the treatment of asymptomatic carriers and in the eradication of spores, which are the main transmissible form of this organism.37,38 In addition, 20% of patients treated with metronidazole or vancomycin will have a symptomatic recurrence when treatment is discontinued, and are at increased risk for multiple recurrences.12 Patients who suffer two recurrences have an approximate risk of 65% for further recurrence of CDI, and this form of CDI is a substantial clinical management problem.37 The limitations of conventional therapy, the incongruity of treating antibiotic-associated diarrhea with additional antibiotics, the rapid increase in the incidence of CDI over the past decade, and the risk of inducing antibiotic resistance, require the development of new strategies to limit the impact of this opportunistic pathogen.15
In conclusion, the results of this study added further elements to the existing but limited data regarding CDI in Argentina and Mexico. Caution should be exercised when extrapolating these data to the country level or to Latin America as a whole due to the epidemiological heterogeneity of the disease within and across countries in that region. However, these data confirm the presence of C. difficile in both countries and support the need to further assess the overall burden of the disease in this region.
FundingThe study sponsor, Sanofi Pasteur, was involved in the trial design, the management and analysis of data and in the decision to publish. This manuscript was prepared with the assistance of Nicolas Voirin, funded by Sanofi Pasteur.
Conflicts of interestThe authors declare no conflicts of interest.
The authors acknowledge with thanks the valuable contribution of Dr Dale N. Gerding, VA Chicago Health Care System, Chicago, IL, USA, in charge of the laboratory where cytotoxicity testing was conducted. The authors also wish to acknowledge the contribution of Dr Enrique Rafael Ortiz Garcia from the Regional Hospital Adolfo López Mateos in Toluca (State of México).