in Brazil, chronic hepatitis C in patients coinfected with the human immunodeficiency virus (HIV) is treated with pegylated interferon (Peg-IFN) and ribavirin (RBV). However, few studies have evaluated the effectiveness of this treatment in this particular population. The identification of the factors that predict sustained virological response (SVR) under current clinical practice would enable clinicians to more accurately estimate the probability of achieving an SVR and therefore utilize the appropriate therapeutics, especially in the era of direct-acting antiviral (DAA) agents.
Aimsthe primary aim of our study was to determine the SVR rate under current clinical practice. The secondary aims were as follows: (1) to determine the factors before and during treatment that predict SVR; and (2) to identify the causes of treatment interruption.
Methodswithin a cohort of HIV/hepatitis C virus (HCV)-coinfected patients in Brazil, we performed a retrospective analysis of those individuals treated with Peg-IFN and RBV.
Resultsamong the 382 analyzed patients, SVR was observed in 118 [30.9% (95% confidence interval (CI): 26.3–35.8)], which included 25.9% (75/289) of the patients with genotypes 1 and 4 and 48.2% (41/85) of those with genotypes 2 and 3. After multivariate analyses the independent positive predictors for SVR after treatment for chronic hepatitis C with Peg-IFN and RBV were: absence of an AIDS-defining illness (p=0.001), HCV viral load lower than 600,000IU/mL at the onset of treatment (p=0.003), higher liver enzyme levels (p=0.039) at baseline, infection with genotypes 2 or 3 (p=0.003), and no transient treatment interruption (p=0.001).
The treatment was interrupted in 25.6% (98/382) of the patients because of adverse events (11.3%, 43/382), virologic failure (7.8%, 30/382), and dropout (6.5%, 43/382). The main adverse events were cytopenia and psychiatric disorders.
Conclusionsin our Brazilian case series, the SVR rate under current clinical practice conditions was similar to that reported in other studies. There was a correlation between an SVR and being infected by genotypes 2 and 3, low viral load, high ALT levels at the onset of treatment, and absence of an AIDS-defining illness. Cytopenia and psychiatric disorders were the major causes of treatment interruption. Efforts should be focused on optimizing management of side effects and counseling to improve adherence and to keep patients on treatment.
Therapeutic decisions regarding the treatment of hepatitis C with pegylated interferon (Peg-IFN) and ribavirin (RBV) in patients coinfected with the human immunodeficiency virus (HIV) are complex. Numerous factors must be considered, such as the status of the HIV infection, the stage of liver fibrosis, the probability of attaining sustained virologic response (SVR), potential treatment risks, and any comorbidities. The treatment regimen consisting of Peg-IFN and RBV has a high rate of ineligibility,1 an increased frequency of adverse events, lower rates of SVR, and more relapses among the HIV/hepatitis C virus (HCV)-coinfected population compared with HCV mono-infected patients.2 The development and utilization of direct-acting antiviral (DAA) agents should advance the treatment paradigms.
Currently, Peg-IFN and RBV, in combination with new oral DAA agents, remain the basis of the therapeutic regimens utilized to treat HCV genotype 1 and the other genotypes. Treating chronic hepatitis C with DAA agents against HCV in HIV/HCV-coinfected patients faces many challenges, including drug interactions with antiretroviral (ARV) agents,3,4 increased toxicity due to the combination therapy with ARVs, rapid selection of HCV-resistant mutants, treatment compliance with multiple medications, and excessive pill burden.5 HCV protease inhibitors are approved for clinical use in HCV-mono-infected patients.6–9 Recently published data reported that HIV/HCV-coinfected patients treated with first wave protease inhibitors (telaprevir and boceprevir),10,11 second wave protease inhibitors (simeprevir and faldaprevir)12,13 and polymerase inhibitor (sofosbuvir)14,15 had higher SVR rates compared with those treated with Peg-IFN and RBV. Based on these data, the international guidelines recommend new DAA for the treatment of HIV/HCV-coinfected patients.16–19
Several studies have analyzed the efficacy and safety of combining Peg-IFN and RBV to treat hepatitis C in HIV-coinfected patients. Although in randomized studies the SVR rate varied from 26 to 55%,20–23 the response rate reported in observational studies was lower, ranging from 12 to 21%.24–27
The current recommendations for the treatment of chronic hepatitis C with Peg-IFN and RBV are based on the above-mentioned randomized studies, which included highly selective cohorts of patients without clinically significant comorbidities and with supervised adherence, such as assistance at reference centers, and experienced physicians. The aim of this study was to assess the effectiveness of treatment with Peg-IFN and RBV under typical conditions, i.e., not as part of a research protocol and in a population of patients treated at several Brazilian centers, by determining the SVR rate and the related factors, and the frequency and causes of treatment interruption.
MethodsDesign and selection of patientsThis study was a retrospective, observational, and non-probabilistic sampling study based on a cohort that included 12 centers at various locations in Brazil. All the HIV/HCV-coinfected patients who were treated with at least one dose of Peg-IFN and RBV (48-week regimen) between January 2005 and June 2008 and were assisted at these centers were included (intention-to-treat analysis). The treatment decisions at the centers were based on the guidelines of the Brazilian Health Ministry (which followed the international recommendations at the time) and were at the discretion of the attending physicians. This study was approved by the research ethics committees at all of the participating centers.
Assessment of effectivenessEffectiveness was determined based on virological response at the end of the treatment (HCV-RNA undetectable or <50IU/mL) and on SVR (HCV-RNA undetectable or <50IU/mL 24 weeks after the end of the treatment). Only intention-to-treat analysis was considered.
Analyzed variablesThe variables selected for analysis were grouped into several categories: variables related to the patients (age, gender, and weight), variables related to HCV infection [level of alanine transaminase (ALT) before treatment, quantitative HCV-RNA measurements before treatment, HCV genotype, and pattern of liver fibrosis], variables related to HCV treatment [number of treatments received; type, dose, and length of Peg-IFN and RBV treatment; and frequency and causes of transient interruption (interruption of RBV and/or Peg-IFN, up to a maximum of two weeks) and treatment discontinuation (interruption of RBV and/or Peg-IFN treatment prior to the completion of the scheduled 48 weeks)], and variables related to HIV infection [history of acquired immunodeficiency syndrome (AIDS)-defining illness, length of antiretroviral therapy (ART), use of zidovudine (AZT), number of CD4+ T cells before treatment, and number of nadir CD4+ T cells].
We reported the liver biopsy results (the degree of inflammatory activity and the stage of fibrosis) as defined by the METAVIR Cooperative Study Group.28 In addition, the degree of steatosis and siderosis were determined by histological examination of the liver.
Statistical analysisThe qualitative variables were expressed as frequencies and percentages, and the quantitative variables were reported as measures of central tendency. For the binary outcomes, the correlation between exposure and outcome was estimated using the prevalence ratio (PR).29,30 The variables with a p-value<0.25 by univariate analysis were selected for a multiple analysis of variance using a Cox regression model with robust variance.31 The variables with a p-value<0.05 in the multiple analysis remained in the final model. Finally, the PR of each such variable was estimated together with the corresponding confidence interval (95% CI) at a 5% descriptive level.
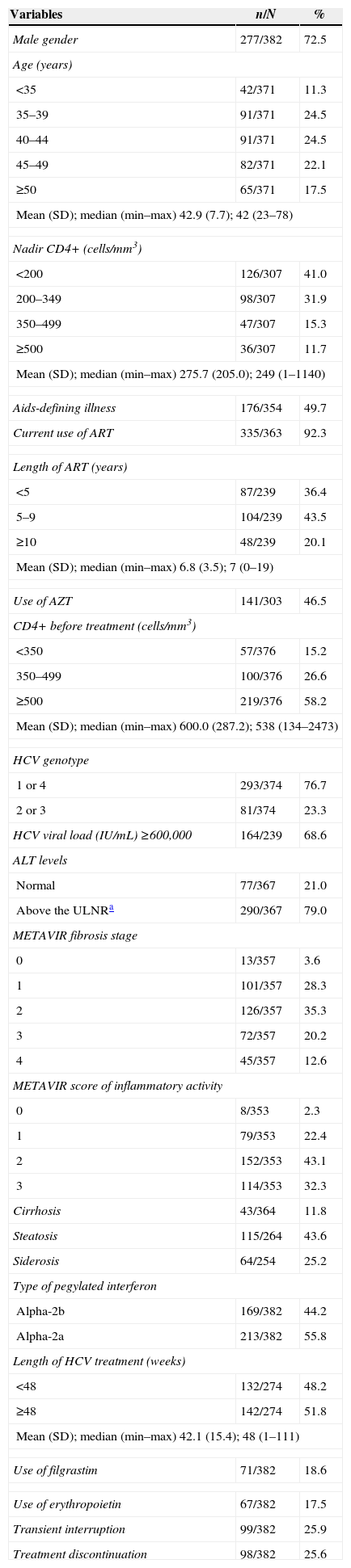
ResultsCharacterization of the population sampleThis study included 382 HIV-HCV-coinfected patients from 12 different Brazilian institutions. Most of the patients were male (72.5%), 40 years old or older (64.2%), had no history of AIDS-defining illness (50.3%), were on ART (92.3%) for at least five years (63.6%), and had 500 or more CD4+ T cells/mm3 in the peripheral blood before treatment (58.2%) (Table 1). The average weight of the patients was 68.4kg [standard deviation (SD)=12.4kg].
Baseline characteristics of the study subjects.
| Variables | n/N | % |
|---|---|---|
| Male gender | 277/382 | 72.5 |
| Age (years) | ||
| <35 | 42/371 | 11.3 |
| 35–39 | 91/371 | 24.5 |
| 40–44 | 91/371 | 24.5 |
| 45–49 | 82/371 | 22.1 |
| ≥50 | 65/371 | 17.5 |
| Mean (SD); median (min–max) 42.9 (7.7); 42 (23–78) | ||
| Nadir CD4+ (cells/mm3) | ||
| <200 | 126/307 | 41.0 |
| 200–349 | 98/307 | 31.9 |
| 350–499 | 47/307 | 15.3 |
| ≥500 | 36/307 | 11.7 |
| Mean (SD); median (min–max) 275.7 (205.0); 249 (1–1140) | ||
| Aids-defining illness | 176/354 | 49.7 |
| Current use of ART | 335/363 | 92.3 |
| Length of ART (years) | ||
| <5 | 87/239 | 36.4 |
| 5–9 | 104/239 | 43.5 |
| ≥10 | 48/239 | 20.1 |
| Mean (SD); median (min–max) 6.8 (3.5); 7 (0–19) | ||
| Use of AZT | 141/303 | 46.5 |
| CD4+ before treatment (cells/mm3) | ||
| <350 | 57/376 | 15.2 |
| 350–499 | 100/376 | 26.6 |
| ≥500 | 219/376 | 58.2 |
| Mean (SD); median (min–max) 600.0 (287.2); 538 (134–2473) | ||
| HCV genotype | ||
| 1 or 4 | 293/374 | 76.7 |
| 2 or 3 | 81/374 | 23.3 |
| HCV viral load (IU/mL) ≥600,000 | 164/239 | 68.6 |
| ALT levels | ||
| Normal | 77/367 | 21.0 |
| Above the ULNRa | 290/367 | 79.0 |
| METAVIR fibrosis stage | ||
| 0 | 13/357 | 3.6 |
| 1 | 101/357 | 28.3 |
| 2 | 126/357 | 35.3 |
| 3 | 72/357 | 20.2 |
| 4 | 45/357 | 12.6 |
| METAVIR score of inflammatory activity | ||
| 0 | 8/353 | 2.3 |
| 1 | 79/353 | 22.4 |
| 2 | 152/353 | 43.1 |
| 3 | 114/353 | 32.3 |
| Cirrhosis | 43/364 | 11.8 |
| Steatosis | 115/264 | 43.6 |
| Siderosis | 64/254 | 25.2 |
| Type of pegylated interferon | ||
| Alpha-2b | 169/382 | 44.2 |
| Alpha-2a | 213/382 | 55.8 |
| Length of HCV treatment (weeks) | ||
| <48 | 132/274 | 48.2 |
| ≥48 | 142/274 | 51.8 |
| Mean (SD); median (min–max) 42.1 (15.4); 48 (1–111) | ||
| Use of filgrastim | 71/382 | 18.6 |
| Use of erythropoietin | 67/382 | 17.5 |
| Transient interruption | 99/382 | 25.9 |
| Treatment discontinuation | 98/382 | 25.6 |
Regarding the HCV infection, 76.7% of the patients had genotypes 1 or 4, 68.6% presented with a viral load >600,000IU/mL, and 79.0% exhibited ALT levels above the upper limit of the normal range (Table 1).
According to liver histopathological assessment before treatment, the cohort included patients with advanced fibrosis (F3) (20.2%), cirrhosis (12.6%), moderate or intense inflammatory activity (75.4%), some degree of steatosis (43.6%), and some degree of siderosis (25.2%).
It is noteworthy that 77 (21.5%) patients had previously received anti-HCV treatment consisting of conventional interferon and RBV.
For the current anti-HCV treatment, most patients were given Peg-IFN-alpha-2a (55.8%), and the median length of treatment was 48 weeks (n=274). Overall, 18.6% of the patients received filgrastim, and 17.5% took erythropoietin.
Transient interruption or early treatment discontinuation occurred among 99 (25.9%) and 98 (25.6%) patients, respectively.
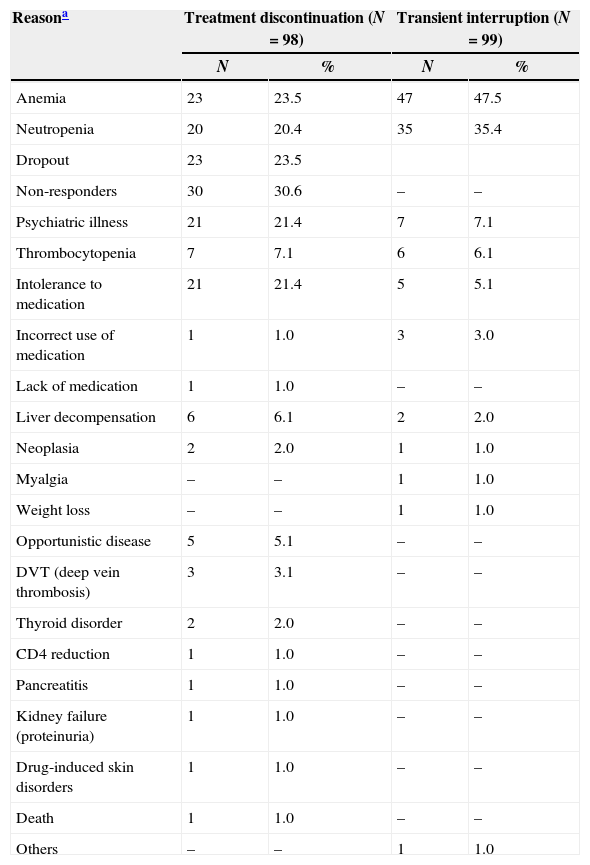
In 25.6% of the patients (98/382), the treatment was discontinued prematurely because of adverse events (11.3%, 43/382), virologic failure (7.8%, 30/382), and dropout (6.5%, 25/382). The most frequent reasons for early treatment discontinuation among 98 patients were as follows: nonresponse to treatment (n=30; 30.6%), anemia (n=23; 23.5%), dropout (n=23; 23.5%), intolerance to the medication (n=21; 21.4%), and psychiatric illness that contra-indicated treatment (n=17; 17.3%). The most frequent reasons for transient interruption included the following: anemia (n=47; 47.5%), neutropenia (n=47; 35.4%), and poor adherence (n=10; 10.1%) (Table 2). Some patients had more than one cause of interruption.
Reasons for transient interruption or early treatment discontinuation.
| Reasona | Treatment discontinuation (N=98) | Transient interruption (N=99) | ||
|---|---|---|---|---|
| N | % | N | % | |
| Anemia | 23 | 23.5 | 47 | 47.5 |
| Neutropenia | 20 | 20.4 | 35 | 35.4 |
| Dropout | 23 | 23.5 | ||
| Non-responders | 30 | 30.6 | – | – |
| Psychiatric illness | 21 | 21.4 | 7 | 7.1 |
| Thrombocytopenia | 7 | 7.1 | 6 | 6.1 |
| Intolerance to medication | 21 | 21.4 | 5 | 5.1 |
| Incorrect use of medication | 1 | 1.0 | 3 | 3.0 |
| Lack of medication | 1 | 1.0 | – | – |
| Liver decompensation | 6 | 6.1 | 2 | 2.0 |
| Neoplasia | 2 | 2.0 | 1 | 1.0 |
| Myalgia | – | – | 1 | 1.0 |
| Weight loss | – | – | 1 | 1.0 |
| Opportunistic disease | 5 | 5.1 | – | – |
| DVT (deep vein thrombosis) | 3 | 3.1 | – | – |
| Thyroid disorder | 2 | 2.0 | – | – |
| CD4 reduction | 1 | 1.0 | – | – |
| Pancreatitis | 1 | 1.0 | – | – |
| Kidney failure (proteinuria) | 1 | 1.0 | – | – |
| Drug-induced skin disorders | 1 | 1.0 | – | – |
| Death | 1 | 1.0 | – | – |
| Others | – | – | 1 | 1.0 |
Among the 382 analyzed patients, 118 patients achieved SVR [30.9% (95% CI: 26.3–35.8)]. Among them 25.9% (75/289) had genotypes 1 or 4 and 48.2% (41/85) had genotypes 2 or 3.
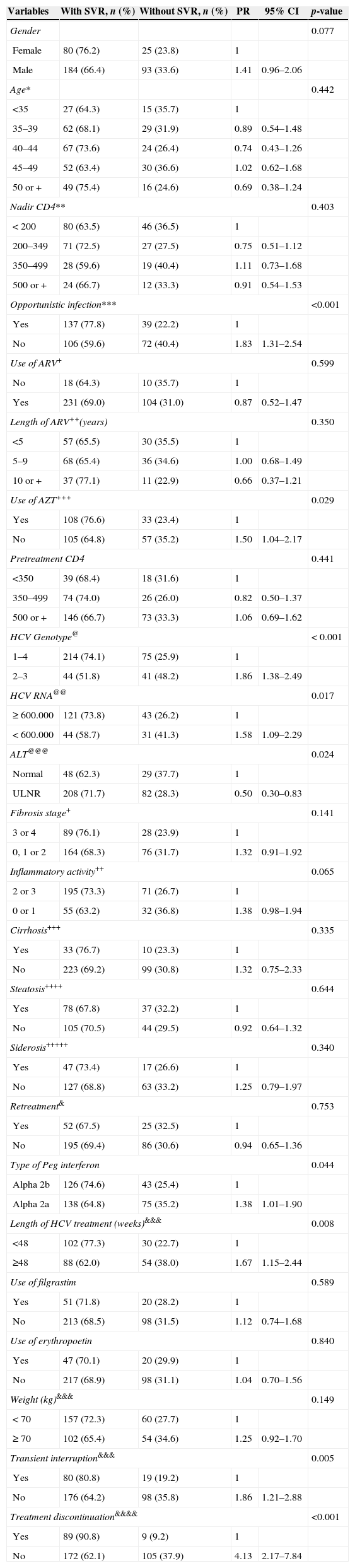
Univariate analysisWe conducted a univariate analysis to test the association of each variable and SVR. SVR was associated to: absence of AIDS-defining illness (p<0.001), no current use of AZT (p=0.029), HCV genotypes 2 or 3 (p<0.001), baseline HCV viral load lower than 600,000IU/mL (p=0.017), higher ALT level at baseline (p=0.024, use of Peg-IFN-alpha-2a (p=0.044), 48-week treatment duration (p=0.008), and no treatment interruption (p=0.005) or discontinuation (p<0.001) (Table 3).
Univariate analysis of the factors associated with SVR.
| Variables | With SVR, n (%) | Without SVR, n (%) | PR | 95% CI | p-value |
|---|---|---|---|---|---|
| Gender | 0.077 | ||||
| Female | 80 (76.2) | 25 (23.8) | 1 | ||
| Male | 184 (66.4) | 93 (33.6) | 1.41 | 0.96–2.06 | |
| Age* | 0.442 | ||||
| <35 | 27 (64.3) | 15 (35.7) | 1 | ||
| 35–39 | 62 (68.1) | 29 (31.9) | 0.89 | 0.54–1.48 | |
| 40–44 | 67 (73.6) | 24 (26.4) | 0.74 | 0.43–1.26 | |
| 45–49 | 52 (63.4) | 30 (36.6) | 1.02 | 0.62–1.68 | |
| 50 or + | 49 (75.4) | 16 (24.6) | 0.69 | 0.38–1.24 | |
| Nadir CD4** | 0.403 | ||||
| < 200 | 80 (63.5) | 46 (36.5) | 1 | ||
| 200–349 | 71 (72.5) | 27 (27.5) | 0.75 | 0.51–1.12 | |
| 350–499 | 28 (59.6) | 19 (40.4) | 1.11 | 0.73–1.68 | |
| 500 or + | 24 (66.7) | 12 (33.3) | 0.91 | 0.54–1.53 | |
| Opportunistic infection*** | <0.001 | ||||
| Yes | 137 (77.8) | 39 (22.2) | 1 | ||
| No | 106 (59.6) | 72 (40.4) | 1.83 | 1.31–2.54 | |
| Use of ARV+ | 0.599 | ||||
| No | 18 (64.3) | 10 (35.7) | 1 | ||
| Yes | 231 (69.0) | 104 (31.0) | 0.87 | 0.52–1.47 | |
| Length of ARV++(years) | 0.350 | ||||
| <5 | 57 (65.5) | 30 (35.5) | 1 | ||
| 5–9 | 68 (65.4) | 36 (34.6) | 1.00 | 0.68–1.49 | |
| 10 or + | 37 (77.1) | 11 (22.9) | 0.66 | 0.37–1.21 | |
| Use of AZT+++ | 0.029 | ||||
| Yes | 108 (76.6) | 33 (23.4) | 1 | ||
| No | 105 (64.8) | 57 (35.2) | 1.50 | 1.04–2.17 | |
| Pretreatment CD4 | 0.441 | ||||
| <350 | 39 (68.4) | 18 (31.6) | 1 | ||
| 350–499 | 74 (74.0) | 26 (26.0) | 0.82 | 0.50–1.37 | |
| 500 or + | 146 (66.7) | 73 (33.3) | 1.06 | 0.69–1.62 | |
| HCV Genotype@ | < 0.001 | ||||
| 1–4 | 214 (74.1) | 75 (25.9) | 1 | ||
| 2–3 | 44 (51.8) | 41 (48.2) | 1.86 | 1.38–2.49 | |
| HCV RNA@@ | 0.017 | ||||
| ≥ 600.000 | 121 (73.8) | 43 (26.2) | 1 | ||
| < 600.000 | 44 (58.7) | 31 (41.3) | 1.58 | 1.09–2.29 | |
| ALT@@@ | 0.024 | ||||
| Normal | 48 (62.3) | 29 (37.7) | 1 | ||
| ULNR | 208 (71.7) | 82 (28.3) | 0.50 | 0.30–0.83 | |
| Fibrosis stage+ | 0.141 | ||||
| 3 or 4 | 89 (76.1) | 28 (23.9) | 1 | ||
| 0, 1 or 2 | 164 (68.3) | 76 (31.7) | 1.32 | 0.91–1.92 | |
| Inflammatory activity++ | 0.065 | ||||
| 2 or 3 | 195 (73.3) | 71 (26.7) | 1 | ||
| 0 or 1 | 55 (63.2) | 32 (36.8) | 1.38 | 0.98–1.94 | |
| Cirrhosis+++ | 0.335 | ||||
| Yes | 33 (76.7) | 10 (23.3) | 1 | ||
| No | 223 (69.2) | 99 (30.8) | 1.32 | 0.75–2.33 | |
| Steatosis++++ | 0.644 | ||||
| Yes | 78 (67.8) | 37 (32.2) | 1 | ||
| No | 105 (70.5) | 44 (29.5) | 0.92 | 0.64–1.32 | |
| Siderosis+++++ | 0.340 | ||||
| Yes | 47 (73.4) | 17 (26.6) | 1 | ||
| No | 127 (68.8) | 63 (33.2) | 1.25 | 0.79–1.97 | |
| Retreatment& | 0.753 | ||||
| Yes | 52 (67.5) | 25 (32.5) | 1 | ||
| No | 195 (69.4) | 86 (30.6) | 0.94 | 0.65–1.36 | |
| Type of Peg interferon | 0.044 | ||||
| Alpha 2b | 126 (74.6) | 43 (25.4) | 1 | ||
| Alpha 2a | 138 (64.8) | 75 (35.2) | 1.38 | 1.01–1.90 | |
| Length of HCV treatment (weeks)&&& | 0.008 | ||||
| <48 | 102 (77.3) | 30 (22.7) | 1 | ||
| ≥48 | 88 (62.0) | 54 (38.0) | 1.67 | 1.15–2.44 | |
| Use of filgrastim | 0.589 | ||||
| Yes | 51 (71.8) | 20 (28.2) | 1 | ||
| No | 213 (68.5) | 98 (31.5) | 1.12 | 0.74–1.68 | |
| Use of erythropoetin | 0.840 | ||||
| Yes | 47 (70.1) | 20 (29.9) | 1 | ||
| No | 217 (68.9) | 98 (31.1) | 1.04 | 0.70–1.56 | |
| Weight (kg)&&& | 0.149 | ||||
| < 70 | 157 (72.3) | 60 (27.7) | 1 | ||
| ≥ 70 | 102 (65.4) | 54 (34.6) | 1.25 | 0.92–1.70 | |
| Transient interruption&&& | 0.005 | ||||
| Yes | 80 (80.8) | 19 (19.2) | 1 | ||
| No | 176 (64.2) | 98 (35.8) | 1.86 | 1.21–2.88 | |
| Treatment discontinuation&&&& | <0.001 | ||||
| Yes | 89 (90.8) | 9 (9.2) | 1 | ||
| No | 172 (62.1) | 105 (37.9) | 4.13 | 2.17–7.84 | |
Missing values: (*)11; (**)75; (***)28; (****)19; (#)143; (##)79; (###)6; (@)8; (@@)143; (@@@)15; (+)25; (++)29; (+++)17; (++++)118; (+++++)128; (&)24; (&&)108; (&&&)9; (&&&&)7.
PR: prevalence ratio; AZT: zidovudine.
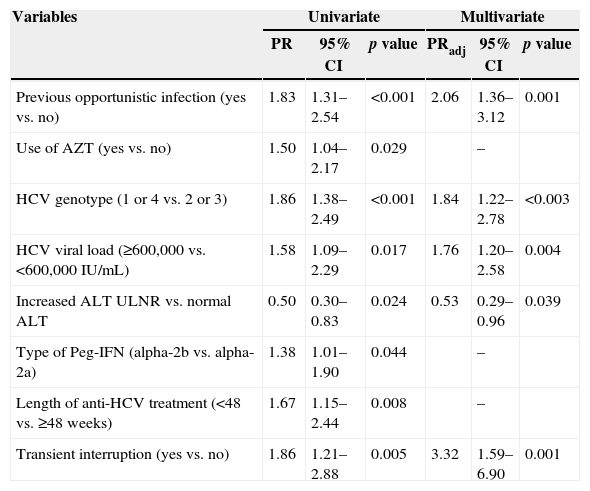
According to the multivariate analysis the following variables correlated with SVR: absence of AIDS-defining illness (p=0.001), HCV viral load lower than 600,000IU/mL at the onset of treatment (p=0.003), higher liver enzyme levels (p=0.039), infection with genotypes 2 or 3 (p<0.003), and no transient treatment interruption (p=0.001) (Table 4).
Variables elected from uni- to multivariate analyses of the factors associated with SVR.
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| PR | 95% CI | p value | PRadj | 95% CI | p value | |
| Previous opportunistic infection (yes vs. no) | 1.83 | 1.31–2.54 | <0.001 | 2.06 | 1.36–3.12 | 0.001 |
| Use of AZT (yes vs. no) | 1.50 | 1.04–2.17 | 0.029 | – | ||
| HCV genotype (1 or 4 vs. 2 or 3) | 1.86 | 1.38–2.49 | <0.001 | 1.84 | 1.22–2.78 | <0.003 |
| HCV viral load (≥600,000 vs. <600,000IU/mL) | 1.58 | 1.09–2.29 | 0.017 | 1.76 | 1.20–2.58 | 0.004 |
| Increased ALT ULNR vs. normal ALT | 0.50 | 0.30–0.83 | 0.024 | 0.53 | 0.29–0.96 | 0.039 |
| Type of Peg-IFN (alpha-2b vs. alpha-2a) | 1.38 | 1.01–1.90 | 0.044 | – | ||
| Length of anti-HCV treatment (<48 vs. ≥48 weeks) | 1.67 | 1.15–2.44 | 0.008 | – | ||
| Transient interruption (yes vs. no) | 1.86 | 1.21–2.88 | 0.005 | 3.32 | 1.59–6.90 | 0.001 |
PR: prevalence ratio; PRadj: adjusted prevalence ratio.
According to our data, infection with genotypes 2 or 3, low pre-treatment HCV viral load, and higher transaminases at baseline were independent positive predictors of SVR. These factors were identified in previous studies, and our study confirms their relevance in clinical practice.32–36 In addition, a previous history of opportunistic infections and transient treatment interruption robustly correlated with treatment failure, which possibly reflects previous more intense impairment of immune system and problems with adverse events and adherence, respectively. The identification of these factors is important to estimate the chance of SVR before and during treatment.
Anemia was the primary cause for interrupting anti-HCV treatment (23.5%) and also stood out as the main cause of transient treatment interruption (47.5%). Interestingly, 46.5% of the patients included in our study took zidovudine (AZT) at some point of treatment. Currently, AZT is not recommended to be used in combination with RBV because of the high risk of anemia, which necessitates reducing or interrupting RBV. Along with previously published data, our results corroborate the evidence indicating that the combination of AZT and RBV is not appropriate.
Our study also identified psychiatric disorder as an important cause of treatment interruption (17.3%), which highlights the need for more adequate and specific interventions concerning the mental health of the target population to improve treatment adherence. Other important reasons for discontinuation of treatment were neutropenia (20.4%), thrombocytopenia (7.1%), liver decompensation (6.1%), and opportunistic disease (5.1%).
The high rate of treatment interruption in our study is a particular concern in DAAs era. Even with important increase of SVR rates with these new drugs, interferon and ribavirin will be used, particularly in resource-poor settings due to higher costs of interferon free regimens. Using telaprevir or boceprevir will increase the incidence of severe adverse events. This situation can impact on adherence and effectiveness, especially in real-life HCV/HIV coinfected patients, where cases tend to be more severe. It is clear that we need more affordable regimens with interferon and without interferon to increase the access of patients to treatment.
According to results of univariate analysis, premature treatment interruption and duration of anti-HCV treatment correlated with SVR. Nevertheless, we opted not to include those variables in the multivariate analysis because we considered them to be co-linear and a reflection of transient treatment interruptions stemming from virologic failure, adverse events, or dropout. It is clear that the probability of curing chronic hepatitis C is reduced under these circumstances.
Our study was a retrospective, multicenter cohort of HIV/HCV-coinfected patients followed in clinical practice settings at 12 Brazilian centers. Although retrospective studies cannot replace prospective randomized studies, they represent an important source of data on treatments in realistic settings. In particular, such studies provide an opportunity to establish whether the success rates, SVR in this case (efficacy), reported in randomized clinical trials extend to typical treatment scenarios where the patients are exposed to factors that are not assessed in randomized clinical trials (effectiveness). This information is important in pinpointing the probability of therapeutic success for each patient and guiding the population-based decision-making by the health authorities. Therefore, observational studies should be conducted immediately following the conclusion of randomized clinical trials to extend the results obtained under ideal conditions to typical settings.37
Our study had several limitations. First, we were not able to establish how many HIV/HCV-coinfected patients were originally screened, i.e., were considered to be eligible for treatment, or refused treatment. This consideration is critical because we cannot rule out the occurrence of selection bias, which may have favored the inclusion of patients with higher probability of achieving SVR.
Second, because our study was retrospective, we did not have access to all of the patients’ clinical data regarding the analyzed variables. The missing data may have impacted the results. To minimize the effect of this bias on our results, the loss of data was not higher than 10% for any of the variables included in the analysis. This fact reflects the difference between randomized clinical trials, where several parameters are systematically collected, and typical conditions, where only the most relevant parameters needed to monitor treatments are routinely collected.
Third, because our study was retrospective, treatment adherence could not be monitored. However, retrospective studies have the advantage of assessing realistic clinical practice (effectiveness) without the potential bias created by changes in behavior that occur in prospective studies because of the awareness of being under observation (Hawthorne effect).38
In conclusion, in our study patients were treated with Peg-IFN and RBV, and the following were identified as predictors of SVR: HCV genotypes 2 or 3, low HCV viral load, high ALT levels at the onset of treatment, no previous history of an AIDS-defining illness and no transient treatment interruption. The identification of these factors may help clinicians to estimate the probability of achieving an SVR and therefore make more appropriate therapeutic decisions. The use of HCV new DAA in HIV/HCV-coinfected patients is currently indicated. With these new drugs, the efficacy of treating HIV/HCV-coinfected patients should increase and with less contra-indications and best tolerance, we will enhance the access to treatment final effectiveness. The potential repercussions in realistic treatment settings are unknown, as the pharmacoeconomic impact, particularly in resource limited situation. In many cases, success will certainly depend on the appropriate management of the basic Peg-IFN and RBV combination therapy.
Conflicts of interestThe authors have no conflict of interest to declare.