the impact of human immunodeficiency virus type 1 (HIV-1) on lung function is well known and associated with a reduction in pulmonary ventilation. Moreover, the use of highly active antiretroviral therapy has been associated with mitochondrial dysfunction and decreased muscle strength. However, there is scarce information about the factors associated with inspiratory muscle weakness in these patients.
Objectivethe purpose of the present study was to investigate the factors associated with inspiratory muscle weakness in patients with HIV-1.
Methodstwo-hundred fifty seven patients with HIV-1 were screened and categorized into two groups: (1) IMW+ (n=142) and (2) IMW− (n=115). Lung function (FEV1, FVC and FEV1/FVC), maximum inspiratory pressure, distance on the six-minute walk test and CD4 cell count were assessed.
Resultsthe mean duration of HIV infection was similar in the two groups. The following variables were significantly different between groups: mean duration of highly active antiretroviral therapy (81±12 in IMW+ versus 38±13 months in IMW−; p=0.01), and CD4 cell count (327±88 in IMW+ versus 637±97cells/mm3 in IMW−; p=0.02). IMW+ presented reduced lung function (FEV1, FVC, FEV1/FVC).
Conclusionpatients with IMW+ had lower distance on the six-minute walk test in comparison to the IMW− group. The duration of highly active antiretroviral therapy, distance traveled on the 6MWT and CD4 count were determinants of IMW in patients with HIV.
The use of highly-active antiretroviral therapy (HAART) has improved the prognosis of patients infected by the human immunodeficiency virus type 1 (HIV-1).1–4 A previous study has demonstrated that such individuals exhibit progressive skeletal muscle dysfunction affecting exercise performance.5 However, other studies suggest that mitochondrial function may be adversely affected by the biochemical changes that occur in HIV-1 infection6 or by the use of HAART.7 These factors help explain the inspiratory muscle weakness (IMW) found in patients with HIV-1, as evidenced by reduced maximum inspiratory pressure (MIP <70% of predicted value).8–11
The mechanisms responsible for limited exercise capacity include abnormalities in the central hemodynamics (sluggish blood flow and faster oxygen extraction in peripheral tissues) as well as autonomic, vascular and respiratory responses to exercise.11 However, studies have shown that even asymptomatic patients with HIV-1 exhibit generalized wasting and significant loss of functional capacity, as demonstrated by a shorter distance traveled on the six-minute walk test (6MWT).5,12
Other factors linked to physical de-conditioning include the chronic nature of the disease, anemia, diabetes, infection and peripheral oxidative dysfunction related to the use of nucleoside reverse transcriptase inhibitors.8,10 These factors result in exaggerated ventilatory response to exercise, with reduced lung compliance10 and progressive recruitment of inspiratory and expiratory muscles, resulting in inspiratory muscle fatigue during exercise. The inspiratory muscles may also be adversely affected by changes in and resistance to contractile properties13,14 due to chronic oxidative stress and infection.15
According to Schulz et al.16 impaired respiratory muscle function may contribute to the unexplained shortness of breath found in patients with HIV. However, no previous study has addressed the determinants of IMW in such individuals. Thus, the aim of the present study was to investigate possible factors associated with IMW in patients with HIV.
Material and methodsSubjectsA comparative study was carried out involving 257 patients with HIV-1 receiving HAART and physically inactive for at least six months. Patients were recruited from a national reference center for acquired immunodeficiency syndrome (AIDS) in Brazil. The inclusion criteria were a diagnosis of HIV infection, clinical stability (without hemodynamic instability or presence of opportunistic disease) and no change in antiretroviral regimen in the previous three months. The exclusion criteria were unstable angina, myocardial infarction, chronic lung disease, or cardiac surgery in the previous three months; chronic metabolic or orthopedic condition treated with steroids or hormones; undergoing chemotherapy for cancer; history of lung disease (forced vital capacity [FVC] <80% of predicted value and/or forced expiratory volume in 1s [FEV1] <70% of predicted value); history of exercise-induced asthma; and smoking habit. This study received approval from the Human Research Ethics Committee of the Hospital Clinicas de Porto Alegre (Brazil) under process number 240.254 and all subjects signed a statement of informed consent.
ProtocolThe medical history of eligible patients was recorded and the subjects were submitted to an initial physical examination, resting electrocardiogram, the determination of inspiratory muscle function and the 6MWT. The patients also provided blood samples for fasting glucose, total-, LDL- and HDL-cholesterol, CD4 and CD8 counts. The participants had two came to the laboratory on day 1 and 48h thereafter. The patients had to undergo a protocol for maximal inspiratory muscle strength (MIP) evaluation and were categorized into two groups: (1) with maximal inspiratory muscle dysfunction denominated inspiratory muscle weakness positive (IMW+, MIP <70% of predicted value);11,16,17 and (2) without inspiratory muscle weakness, i.e., with MIP preserved denominated IMW− (MIP >70% of predicted value). The 6MWT was performed three times on each evaluation day for the determination of the maximum distance traveled and reproducibility.
Lung functionLung function assessment was performed using the CPF System (Medical Graphics-MGC, St. Paul, MN), with air flow measured using a calibrated Pitot tube (PreVent, Pneumotach). FVC (in liters), FEV1 (in liters), FEV1/FVC and inspiratory capacity (in liters) were recorded as recommended by the American Thoracic Society.18 The results were expressed as absolute and percentage of predicted value. Inspiratory muscle function testing was performed using a pressure transducer system (POWERbreathe K5, Northfield Road, Southam, Warwickshire, UK). Maximum static inspiratory pressure was determined in deep inspiration starting from residual volume. The highest pressure of six measurements was used for analysis.
Six-Minute Walk TestThe 6MWT was performed in an indoor corridor measuring 25m in length, following recommendations by Guyatt et al.19 The participants were instructed to walk from one end to the other of the corridor as many times as possible in six minutes. At the end of the test, the physical therapist measured the total distance traveled. Reproducibility was determined in a sample of the 257 patients and the difference in distance between the two tests was less than 5%. The maximum distance traveled was used to assess submaximal functional capacity. Patients self-graded their degree of dyspnea during the test using the Borg scale.17
Statistical analysisThe data were analyzed with the Statistical Package for the Social Sciences (version 19.0, SPSS, Chicago, IL). Descriptive data are presented as mean±standard deviation. Data of the two groups were compared by one-way repeated-measures analysis of variance. Post hoc analysis was conducted using either Tukey's test or Fisher's exact test for categorical variables. Pearson's correlation coefficients (r) were calculated to determine factors associated with MIP. Stepwise multiple regression analysis was performed with MIP, duration of HAART, distance traveled on the 6MWT and CD4 cell count.
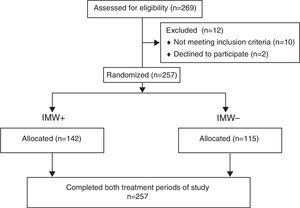
ResultsTwo hundred sixty-nine patients with HIV were evaluated between May 2010 and November 2011. Twelve patients have not met the eligibility criteria and were excluded. Therefore, 257 patients were screened for the study, among whom 142 were categorized as IMW+ (MIP <70% of predicted value) and 115 were IMW− (MIP >70% of predicted value) (Fig. 1).
Table 1 displays the characteristics of the sample. Mean age was 45±11 years. Cholesterol values were not elevated. No abnormalities were found regarding hemodynamics. The IMW+ group had higher fasting glucose. Time elapsed since the diagnosis of HIV infection was not significantly different between groups. IMW+ patients had been under HAART for a longer period of time, had a significantly lower CD4 count, but no difference in the CD8 count, resulting in a lower CD4/CD8 ratio. The lung function was more compromised in IMW+ compared to IMW− (FEV1: 85±6 vs. 93±8% of predicted; p=0.03; FVC: 92±13 vs. 102±9% of predicted; p=0.03), but within normality without differences between FEV1/FVC. The IMW+ group had lower MIP (45±12 vs. 90±17cm H2O; p=0.01) and traveled a shorter distance on the 6MWT (265±38 vs. 452±67m; p=0.01) in comparison to the IMW− group.
Characteristics of participants.
| Characteristics | All patients (n=257) | IMW+ (n=142) | IMW− (n=115) | p-Value |
|---|---|---|---|---|
| Demographic/anthropometric | ||||
| Age (years) | 45±11 | 46.5±11 | 49.2±10 | 0.24 |
| Sex (male/female) | 53/18 | 35/10 | 18/8 | 0.77 |
| BMI (kg/m2) | 23±3 | 23±4 | 25±4 | 0.42 |
| Fasting Glucose (mg/dL) | 99±22 | 91±25 | 86±16 | 0.12 |
| Total cholesterol (mg/dL) | 180±40 | 176±42 | 189±37 | 0.23 |
| HDL-cholesterol (mg/dL) | 43±13 | 46±14 | 39±9 | 0.11 |
| LDL-cholesterol (mg/dL) | 110±45 | 105±34 | 118±37 | 0.29 |
| Hemodynamics | ||||
| MBP (mm Hg) | 90±16 | 98±12 | 90±18 | 0.35 |
| HR (beatsmin−1) | 77±7 | 88±8 | 76±10 | 0.74 |
| Duration of HIV (months) | 122±18 | 104±12 | 111±21 | 0.34 |
| Duration of HAART (months) | 56±27 | 81±12a | 38±13 | 0.01 |
| Virology | ||||
| CD4 count (cells/mm3) | 536±72 | 327±88a | 637±97 | 0.02 |
| CD8 count (cells/mm3) | 1098±444 | 1086±480 | 1124±358 | 0.25 |
| CD4/CD8 ratio | 0.48±0.18 | 0.31±0.04a | 0.56±0.09 | 0.01 |
| CD4 nadir (cells/mm3) | ||||
| Median (interquartile range) | 245 (140–368) | 90 (19–144)a | 270 (159–354) | 0.03 |
| Viral load (log) | 2.1 (1.7–3.5) | 2.5 (1.8–3.6) | 2.1 (1.5–3.4) | 0.55 |
| Viral load (≤50cells/mm3; %) | 71 | 67b | 92 | 0.04 |
| Lung function | ||||
| FEV1 (L) | 3.02±0.45 | 2.66±0.46a | 2.88±0.67 | 0.02 |
| FEV1 (% of predicted) | 95±10 | 85±6a | 93±8 | 0.03 |
| FVC (% of predicted) | 101±12 | 92±13a | 102±9 | 0.03 |
| FEV1/FVC | 94±5 | 92±8 | 91±9 | 0.67 |
| Inspiratory muscle function | ||||
| MIP (cm H2O) | 80±12 | 45±12a | 90±17 | 0.01 |
| MIP (% predicted) | 77±11 | 46±14 | 89±12 | 0.01 |
| Exercise | ||||
| 6MWD (m) | 445±65 | 265±38a | 452±67 | 0.01 |
| 6MWD (% of predicted) | 95±22 | 45±18a | 91±12 | 0.01 |
Continuous variables expressed as mean±standard deviation; categorical variables expressed using chi-square or Fisher's exact test.
BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MBP, mean blood pressure; HR, heart rate; FEV1, forced expiratory volume in 1st min; FVC, forced vital capacity; FEV1/FVC, forced expiratory volume in 1st min to forced vital capacity ratio; MIP, maximal inspiratory pressure; 6MWD, distance traveled on six-minute walk test; HAART, highly active antiretroviral therapy.
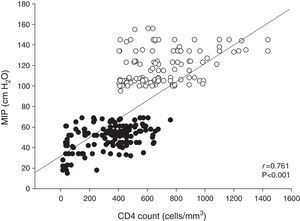
The therapeutic regimens consisted of two nucleoside analog reverse transcriptase inhibitors plus one protease inhibitor, with or without low-dose ritonavir (25%), or a non-nucleoside reverse transcriptase inhibitor (75%). The most common HAART combination in the IMW+ group was zidovudine, lamivudine and efavirenz, which differed from that of patients in the IMW− group. MIP was closely associated with the CD4 cell count in all patients (r=0.761; p<0.001; Fig. 2). In the stepwise multiple regression analysis (Table 2), the CD4 count was a significant determinant of 6MWT. The total variance explained by this model was 56% (p<0.001).
Results of stepwise multiple regression analysis of maximum inspiratory pressure.
| Dependent variable | Independent variable | β | ra |
|---|---|---|---|
| 6MWD | HAART duration | −0.62 | 0.68 |
| MIP | 0.66 | ||
| CD4 count | 0.78 |
MIP, maximum inspiratory pressure; HAART duration, duration of highly active antiretroviral therapy; 6MWD, distance on six-minute walk test; β, standardized partial regression coefficient; r, multiple correlation coefficient.
Table 3 displays the CD4 cell counts in the IMW+ and IMW− groups. Eighty-eight patients in the IMW+ group had <200cells/mm3 and 54 had >200cells/mm3. In the IMW− group, 49 patients had <200cells/mm3 and 66 had >200cells/mm3. Interestingly, the combination of IMW and CD4 count <200cells/mm3 altered pulmonary function, inspiratory muscle strength and distance traveled on the 6MWT in comparison to the absence of IMW and CD4 count >200cells/mm3.
Comparative assessment of pulmonary function, inspiratory muscle strength and distance walked in different groups according to CD4 count.
| IMW+ | IMW− | |||
|---|---|---|---|---|
| CD4 <200 (n=88) | CD4 >200 (n=54) | CD4 <200 (n=49) | CD4 >200 (n=66) | |
| FEV1(L) | 1.88±0.66a,b | 2.58±0.45 | 2.88±0.49c | 3.01±0.56 |
| FEV1(% of predicted) | 83±5a | 90±8 | 95±12 | 96±9 |
| FVC (% of predicted) | 95±12 | 95±7 | 98±8 | 102±12 |
| FEV1/FVC | 88±6a,b | 94±5 | 96±5c | 94±8 |
| MIP (cm H2O) | 45±12a,b | 68±15 | 80±13c | 97±20 |
| MIP (% of predicted) | 46±14a,b | 69±12 | 82±18 | 98±19 |
| 6MWD (m) | 265±38a,b | 301±44 | 349±49c | 452±67 |
| 6MWD (% predicted) | 45±18a,b | 52±14 | 81±27 | 95±29 |
| Viral load (%) | ||||
| ≤50 | 41a | 69 | 60 | 83 |
Data expressed as mean±standard deviation.
IM, inspiratory muscle weakness; FEV1, forced expiratory volume in 1st min; FVC, forced vital capacity; FEV1/FVC, forced expiratory volume in 1st min to forced vital capacity ratio; MIP, maximal inspiratory pressure; 6MWD, distance traveled on six-minute walk test.
The present study is the first to describe the determinants of inspiratory muscle weakness (IMW) in patients with HIV. Inspiratory muscle dysfunction is related to the severity of HIV infection.16,20 The present data reveal that 55% of HIV-infected patients presented IMW in a sample of clinically stable patients. This finding is compatible with previous studies.16,21 However, in contrast to the present study, these studies had smaller sample sizes.
IMW was striking in the patients analyzed. This response is dependent on hemodynamic and clinical variables as well as changes in diaphragm morphology.11 Moreover, the longer duration of HAART in IMW+ patients, associated with both the distance traveled on the 6MWT and CD4 cell count, might have caused higher toxicity in these patients compared to IMW− patients. However, the effects of HIV infection on respiratory muscles function have not been well characterized in the literature. Our study has shown that inspiratory muscle dysfunction can lead to a reduction in lung function, even in clinically stable patients (CD4 >200).22 Previous studies have described that such a reduction is associated with lower CD4 count,23,24 which explains the increase in the frequency of respiratory symptoms combined with dyspnea in patients with HIV. Moreover, impaired lung function is considered to be an important predictor of increased respiratory mortality and closely associated with adverse cardiovascular events.25
HIV infection is associated with progressive depletion of CD4T lymphocytes and defective HIV-specific T-cell responses.26 Persistent immune activation plays a central role in driving CD4 T-cell depletion and progression to AIDS.27,28 The authors of the present study believe that severe infection and reactive oxygen species are required for the molecular cascades that eventually produce myofiber atrophy of the diaphragm, which may favor IMW in patients with HIV.
In the present study, MIP was closely correlated with the distance traveled on the 6MWT (r=0.61; p<0.001) and CD4 cell count (r=0.73; p<0.001), independently of IMW, which suggests an important combination with the inflammatory response. Moreover, associations were found between MIP and duration of HAART (r=−0.62; p<0.001) as well as between the distance traveled on the 6MWT and fasting glucose (r=−0.65, p=0.02). Studies have suggested that inadequate glucose control may be linked to inflammation and decreased lung function in individuals with diabetes.29 The authors of the present study agree with the hypothesis that IMW or dysfunction occurs in the course of HIV infection. Moreover, the authors believe that a reduction in CD4 cell count results in IMW and exercise intolerance.
A number of mechanisms have been suggested to explain the reduction in inspiratory muscle strength in patients with HIV, such as reduced cross-sectional area of all types of muscle fiber in the diaphragm and muscles of the ribcage, type I fiber atrophy, changes in the type I to type IIb fiber ratio in the diaphragm,30–32 a reduced number of actin-myosin cross-bridges in the diaphragm,33 and an abnormal intracellular Ca2+ profile.30,31 The same applies for potential mechanisms leading to inspiratory muscle dysfunction, impaired cardiac function,9 a reduction in oxidative enzymes15,34 and impaired mitochondrial replication through the inhibition of mitochondrial DNA gyrase by zidovudine,16,35 which could further compromise the inspiratory muscles. In the present study, the prevalence of IMW coincided with the use of zidovudine. Evidence suggests that zidovudine and tenofovir result in significant myopathy.21,35,36 Although the patients in the present study exhibited IMW, the authors believe that systemic biochemical abnormalities may occur, resulting in inspiratory muscle dysfunction in patients with HIV. Studies have reported a lack of l-carnitine, which limits the intra-mitochondrial transport of long-chain fatty acids, leading to a change in energy metabolism6 and impairment of diaphragm contractions. Studies have also suggested that an impaired oxidation reduction mechanism in muscle may contribute to oxidative stress in the diaphragm.13,14,37–39
Shortness of breath is often associated with exercise intolerance and it has been suggested that IMW may be an important contributing cause to the sensation of dyspnea,16 as well as to a shorter the distance traveled on the 6MWT in patients with HIV. Several studies have reported a relationship between anemia and fatigue in such patients.40,41 The distance traveled on the 6MWT in the present study provides further evidence that infections could result in decreased exercise capacity.
Surprisingly, a CD4 count <200cell/mm3 affected inspiratory muscle strength and the distance traveled on the 6MWT, which supports our hypothesis that infection is an important factor to exercise intolerance in patients with HIV.
The present study has a limitation that should be addressed. No cardiopulmonary exercise test was performed, which would have confirmed exercise intolerance. However, a submaximal exercise text (6MWT) was used.5 The distance traveled on the 6MWT has demonstrated important associations with the loss of muscle mass, generalized wasting and weaker handgrip strength.12,42 These associations have previously been evaluated in patients with chronic heart failure and IMW.11,19 In our study, IMW was assessed by non-invasive technique as described in other published studies. Perhaps we should have used more invasive measures such as tension time diaphragmatic index or twitch Pdi.
The findings of the present study demonstrate that patients with HIV infection have significant exercise limitations resulting from IMW. The underlying pathomechanisms, responses to treatment, and the potential role of IMW as a prognostic marker of respiratory muscle dysfunction merit further investigation.
Conflicts of interestThe author declares no conflicts of interest.
Financial support was partially provided by National Research Council (CNPq, 478063/2010-5) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, PNPD 2818/2011).