Acute bacterial skin and skin structure infections are caused mainly by Gram-positive bacteria which are often treated with intravenous vancomycin, daptomycin, or linezolid, with potential step down to oral linezolid for outpatients. Tedizolid phosphate 200mg once daily treatment for six days demonstrated non-inferior efficacy, with a favourable safety profile, compared with linezolid 600mg twice daily treatment for 10 days in the Phase 3 ESTABLISH-1 and -2 trials. The objective of the current post-hoc analysis of the integrated dataset of ESTABLISH-1 and -2 was to evaluate the efficacy and safety of tedizolid (N=182) vs linezolid (N=171) in patients of Latino origin enrolled into these trials. The baseline demographic characteristics of Latino patients were similar between the two treatment groups. Tedizolid demonstrated comparable efficacy to linezolid at 48–72h in the intent-to-treat population (tedizolid: 80.2% vs linezolid: 81.9%). Sustained clinical success rates were comparable between tedizolid- and linezolid-treated Latino patients at end-of-therapy (tedizolid: 86.8% vs linezolid: 88.9%). Tedizolid phosphate treatment was well tolerated by Latino patients in the safety population with lower abnormal platelet counts at end-of-therapy (tedizolid: 3.4% vs linezolid: 11.3%, p=0.0120) and lower incidence of gastrointestinal adverse events (tedizolid: 16.5% vs linezolid: 23.5%). Population pharmacokinetic analysis suggested that estimated tedizolid exposure measures in Latino patients vs non-Latino patients were similar. These findings demonstrate that tedizolid phosphate 200mg, once daily treatment for six days was efficacious and well tolerated by patients of Latino origin, without warranting dose adjustment.
Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA), are commonly associated with skin and skin structure infections (SSSIs), bacteraemia and nosocomial pneumonia.1–3 Infections due to MRSA may be associated with morbidity and mortality, particularly in the elderly.3 MRSA-related infections are an increasing problem in Latin America,4–6 both in the healthcare environment and in the community. The epidemiology of MRSA is constantly changing; both hospital-acquired (HA) and community-acquired (CA) MRSA circulating clones and their antibiotic resistance profiles vary considerably throughout regions and countries.7 In 2003, the first outbreak of infections involving CA-MRSA strains in Latin America was described in Uruguay and was caused by the Southwest Pacific (SWP) clone/sequence type (ST)-30/SCCmec IVc.8 Furthermore, other clones of CA-MRSA have been isolated in Brazil,9 Argentina,10 Colombia, Ecuador, Venezuela,11 Mexico,12 and Chile.13 A considerable amount of CA-MRSA has also been found among nosocomial isolates, at least in Colombia and Uruguay.14,15
Vancomycin, teicoplanin, daptomycin, tigecycline, linezolid, clindamycin, and ceftaroline are commercially available as parenteral intravenous agents for MRSA infections in many countries in Latin America.16 Despite the broad range of anti-MRSA antibiotics, initial empirical therapy is inappropriate in a large number of patients leading to treatment failures,17 increased healthcare costs,18 and potentially increasing resistance levels.19
Tedizolid phosphate is a novel oxazolidinone antibiotic20 with at least 4-times higher potency than linezolid against Gram-positive bacteria including MRSA, vancomycin-resistant enterococci (VRE), linezolid-resistant cfr+ S. aureus, and Streptococcus pyogenes.21,22 Tedizolid phosphate is converted in vivo by non-specific phosphatases to its active moiety tedizolid (TZD).23 Two pivotal randomised, double-blind, double-dummy, multicentre, controlled, Phase 3 clinical trials (ESTABLISH-1 and ESTABLISH-2) conducted in patients with acute bacterial skin and skin structure infections (ABSSSI; i.e. cellulitis/erysipelas, wound infection and major cutaneous abscess)24,25 demonstrated that tedizolid phosphate, 200mg, once daily (QD) treatment for six days was non-inferior to linezolid (LZD), 600mg, twice daily (BID) treatment for 10 days.26–28 In addition, TZD had an improved tolerability and safety profile compared with LZD, particularly in terms of gastrointestinal (GI) adverse events (AEs) and haematological parameters.26–28
Interethnic pharmacokinetic (PK) differences exist for certain antibacterial agents potentially influencing the efficacy and safety of the antibiotic drug in patients.29 For example, ciprofloxacin metabolism in Brazilian subjects differs from that in other ethnic populations, while tigecycline clearance in young healthy Afro-American subjects is higher than in Caucasian subjects.29 Furthermore, both intrinsic (e.g. genetics, body size and fat distribution) and extrinsic ethnic factors may influence the effects of an investigational drug via altered PK and pharmacodynamics.30–32
The objectives of the current post-hoc analysis were to evaluate the efficacy and safety of TZD vs LZD for the treatment of ABSSSI in patients of Latino origin enrolled into the Phase 3 ESTABLISH studies. Furthermore, the TZD PK profile of these patients was evaluated based on population PK analysis.
MethodsStudy design and treatmentsBoth ESTABLISH-1 (NCT01170221) and ESTABLISH-2 (NCT01421511) were randomised, multicentre, double-blind, double-dummy, active-controlled Phase 3 studies comparing tedizolid phosphate 200mg QD (6-day course followed by 4-day placebo treatment) vs LZD 600mg BID (10-day treatment) for the treatment of patients with ABSSSIs. Patients enrolled into ESTABLISH-1 received exclusively oral (PO) therapy,26 while patients in ESTABLISH-2 received intravenous (IV) therapy with an optional switch to PO therapy when certain criteria were met.27 The integrated dataset of ESTABLISH-1 and ESTABLISH-2 trials was analysed and reported by Shorr et al.28
Ethical approvalNo ethical approval of this post-hoc analysis was required. The ESTABLISH-1 and ESTABLISH-2 studies were conducted in accordance with the 2008 Declaration of Helsinki and all relevant international, European Union, national, and local rules and legislation. Institutional review board or ethics committee approval was obtained at each participating centre and all participants provided written informed consent. A data and safety monitoring board reviewed safety data during the conduct of the study.26,27
Enrolment criteriaPatients (aged ≥18 years old in ESTABLISH-1 and ≥12 years old in ESTABLISH-2) were enrolled in both trials if they had an ABSSSI (cellulitis/erysipelas, wound infection, or major cutaneous abscess) with a minimum lesion surface area of 75cm2; wound infections and abscesses also required the lesion to extend ≥5cm from the margin of the wound or abscess. In addition, patients had at least one local and one systemic sign of infection, and a suspected/documented Gram-positive pathogen at the site of infection.26,27 The institutional review board or equivalent at each study centre approved the trials and all patients provided written informed consent.26,27 Exclusion criteria were previously described by Prokocimer et al.26 and Moran et al.27
Demographic data, disease characteristics, co-medications, comorbidities, race and ethnic origin (Latino or non-Latino), primary cause of ABSSSI, digital photograph of the lesion, lesion size, vital signs, and microbiological specimen information were recorded on case report forms.
Population definitionsThe intent-to-treat population (ITT analysis set) included all randomised patients (including the Latino subpopulation) who were eligible based on the inclusion criteria outlined above.26,27 The clinically evaluable population (CE) included all patients in the ITT analysis set who (1) complied with the study protocol with no major violations, and (2) completed the clinical response outcome assessment at the end-of-therapy (EOT) visit, and (3) had no concomitant systemic antibiotic therapy or topical antibiotic from the first infusion of study drug through to the EOT visit that was potentially effective against the baseline pathogen, except adjunctive aztreonam and/or metronidazole in patients with wound infections. Two CE populations were analysed: the CE-EOT population included ITT patients who had a valid assessment at the 48- to 72-h visit and at the EOT visit, and the CE-post-treatment evaluation (PTE) population included ITT patients who had a valid assessment at the PTE visit. The safety analysis population was comprised of all randomised patients who received at least one dose of the study drug and had a post-treatment assessment.26,27
Efficacy parametersThe primary efficacy endpoint was early clinical response rate at the 48- to 72-h visit. The primary endpoint in the integrated analysis was represented by ≥20% reduction in lesion size compared with baseline.28 Secondary endpoints included: programmatic and investigator-assessed clinical success rates at EOT (Day 11), investigator-assessed sustained clinical success rates at the PTE visit in the intent-to-treat (ITT) population, and also in the CE population.28
Safety parametersSafety was assessed by collecting information on adverse events based on System Organ Class and definitions were given as coded in Medical Dictionary for Regulatory Activities (MedDRA) v13.1. Treatment-emergent AEs (TEAEs) were defined as those that occurred or worsened after the first dose of study drug. In addition, safety assessments included clinical chemistry and haematology laboratory results, vital signs and electrocardiograms, and physical examinations.
Laboratory measurementsBlood samples were collected at randomisation, on Day 1, Day 3, Day 7, EOT (Day 11), and PTE visits for assessments of various haematological, toxicology parameters (e.g. haemoglobin, platelets, neutrophils, bilirubin, creatinine, alanine aminotransferase (ALT), aspartate aminotransferase [AST]), and clinical chemistry analysis.
Population pharmacokinetic analysisIn the ESTABLISH trials, patients’ baseline parameters (height, body weight, body mass index [BMI], ideal body weight [IBW], age, sex, calculated creatinine clearance based on Cockcroft-Gault formula, and total bilirubin level) were collected, and race and ethnicity (Latino vs non-Latino) were also recorded on case report forms.
The final PK model of TZD (a 2-compartment model with sigmoidal absorption, absolute bioavailability, and linear elimination), was used to estimate TZD exposure measures for each patient based on measured concentrations and statistically significant predictors (IBW and total bilirubin), including the area under the concentration–time curve at 0–24h (AUC0–24h), minimum drug concentration (Cmin), and maximum drug concentration (Cmax) on Day 1 and at steady-state.33 This post-hoc population PK analysis of TZD exposure at steady state in Latino patients included summaries of the mean value, standard deviation, median, and range. Boxplots of TZD AUC0–24h, Cmax and Cmin at steady state are also presented.
Statistical analysisThis post-hoc analysis focused on a subpopulation of patients of Latino origin. Data (i.e. efficacy, safety) are expressed as mean±standard deviation (SD). Population PK data are expressed as mean, SD and range, and data are graphically shown as boxplots including median, 25th, 50th, and 75th percentiles, and whiskers as 5th and 95th percentiles. Statistical significance was defined by p<0.05. Post-hoc statistical analysis was descriptive in nature.
ResultsDemographics of Latino patientsIn the integrated analysis of the ESTABLISH trials a total of 1333 patients were randomised and received study drug, 353 of whom were Latinos (26.5%) in the ITT population (TZD N=182; LZD N=171). Of the Latino patients, 85.6% were enrolled from the US/Canada region, 13.3% from the ‘Other’ region, which included Latin America (Argentina, Brazil, and Peru), Australia, New Zealand, and South Africa, and only 1.1% from Europe.
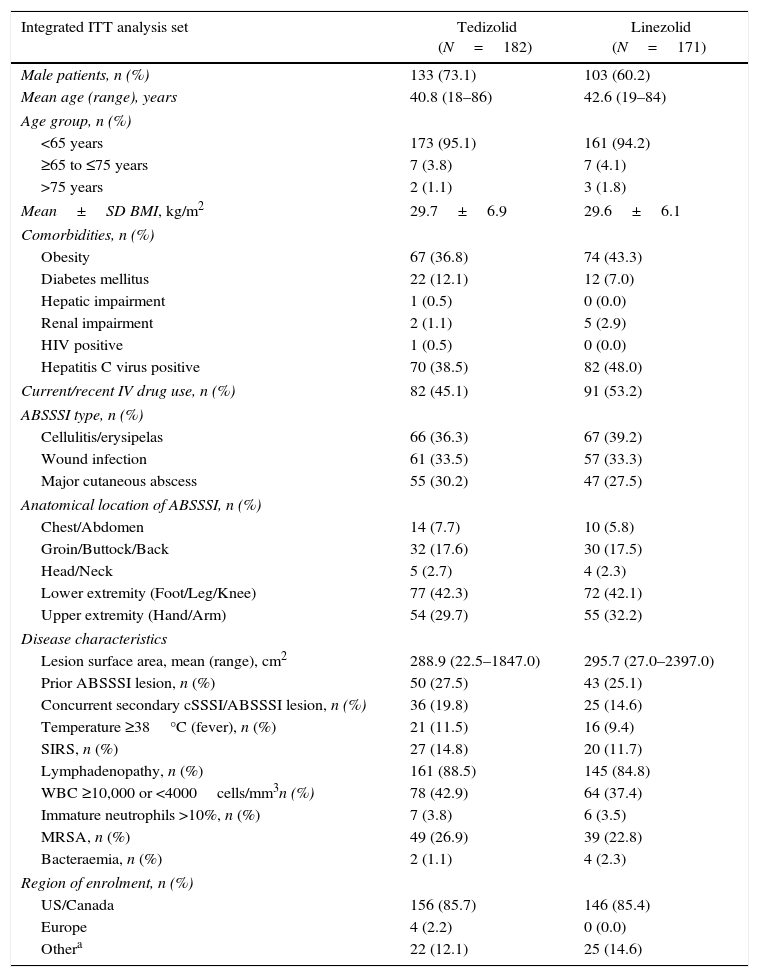
The baseline demographic parameters and disease characteristics of the enrolled Latino patients in ESTABLISH-1 and ESTABLISH-2 were similar between the two treatment groups (Table 1). The proportion of male patients in the TZD arm (73.1%) was slightly higher than in the LZD arm (60.2%), most patients being <65 years old. There was no difference in the BMI of patients treated with TZD (29.7±6.9kg/m2) or LZD (29.6±6.1kg/m2), respectively; on average, 40% of all Latino patients were obese and 9.6% had diabetes mellitus (Table 1).
Baseline demographic parameters and disease characteristics for Latino patients.
| Integrated ITT analysis set | Tedizolid (N=182) | Linezolid (N=171) |
|---|---|---|
| Male patients, n (%) | 133 (73.1) | 103 (60.2) |
| Mean age (range), years | 40.8 (18–86) | 42.6 (19–84) |
| Age group, n (%) | ||
| <65 years | 173 (95.1) | 161 (94.2) |
| ≥65 to ≤75 years | 7 (3.8) | 7 (4.1) |
| >75 years | 2 (1.1) | 3 (1.8) |
| Mean±SD BMI, kg/m2 | 29.7±6.9 | 29.6±6.1 |
| Comorbidities, n (%) | ||
| Obesity | 67 (36.8) | 74 (43.3) |
| Diabetes mellitus | 22 (12.1) | 12 (7.0) |
| Hepatic impairment | 1 (0.5) | 0 (0.0) |
| Renal impairment | 2 (1.1) | 5 (2.9) |
| HIV positive | 1 (0.5) | 0 (0.0) |
| Hepatitis C virus positive | 70 (38.5) | 82 (48.0) |
| Current/recent IV drug use, n (%) | 82 (45.1) | 91 (53.2) |
| ABSSSI type, n (%) | ||
| Cellulitis/erysipelas | 66 (36.3) | 67 (39.2) |
| Wound infection | 61 (33.5) | 57 (33.3) |
| Major cutaneous abscess | 55 (30.2) | 47 (27.5) |
| Anatomical location of ABSSSI, n (%) | ||
| Chest/Abdomen | 14 (7.7) | 10 (5.8) |
| Groin/Buttock/Back | 32 (17.6) | 30 (17.5) |
| Head/Neck | 5 (2.7) | 4 (2.3) |
| Lower extremity (Foot/Leg/Knee) | 77 (42.3) | 72 (42.1) |
| Upper extremity (Hand/Arm) | 54 (29.7) | 55 (32.2) |
| Disease characteristics | ||
| Lesion surface area, mean (range), cm2 | 288.9 (22.5–1847.0) | 295.7 (27.0–2397.0) |
| Prior ABSSSI lesion, n (%) | 50 (27.5) | 43 (25.1) |
| Concurrent secondary cSSSI/ABSSSI lesion, n (%) | 36 (19.8) | 25 (14.6) |
| Temperature ≥38°C (fever), n (%) | 21 (11.5) | 16 (9.4) |
| SIRS, n (%) | 27 (14.8) | 20 (11.7) |
| Lymphadenopathy, n (%) | 161 (88.5) | 145 (84.8) |
| WBC ≥10,000 or <4000cells/mm3n (%) | 78 (42.9) | 64 (37.4) |
| Immature neutrophils >10%, n (%) | 7 (3.8) | 6 (3.5) |
| MRSA, n (%) | 49 (26.9) | 39 (22.8) |
| Bacteraemia, n (%) | 2 (1.1) | 4 (2.3) |
| Region of enrolment, n (%) | ||
| US/Canada | 156 (85.7) | 146 (85.4) |
| Europe | 4 (2.2) | 0 (0.0) |
| Othera | 22 (12.1) | 25 (14.6) |
Other: Latin America (Argentina, Brazil, and Peru), Australia, New Zealand, and South Africa.
ABSSSI, acute bacterial skin and skin structure infection; BMI, body mass index; cSSSI, complicated skin and skin structure infection; HIV, human immunodeficiency virus; ITT, intent-to-treat, IV, intravenous; MRSA, methicillin-resistant Staphylococcus aureus; SD, standard deviation; SIRS, systemic inflammatory response syndrome; US, United States; WBC, white blood cell.
The proportion of patients enrolled with cellulitis, wound infection or major abscess, and the mean lesion size were similar between treatment groups (TZD: 288.9cm2 vs LZD: 295.7cm2) (Table 1). MRSA was the causative Gram-positive pathogen in 26.9% of TZD-treated and 22.8% of LZD-treated patients, respectively.
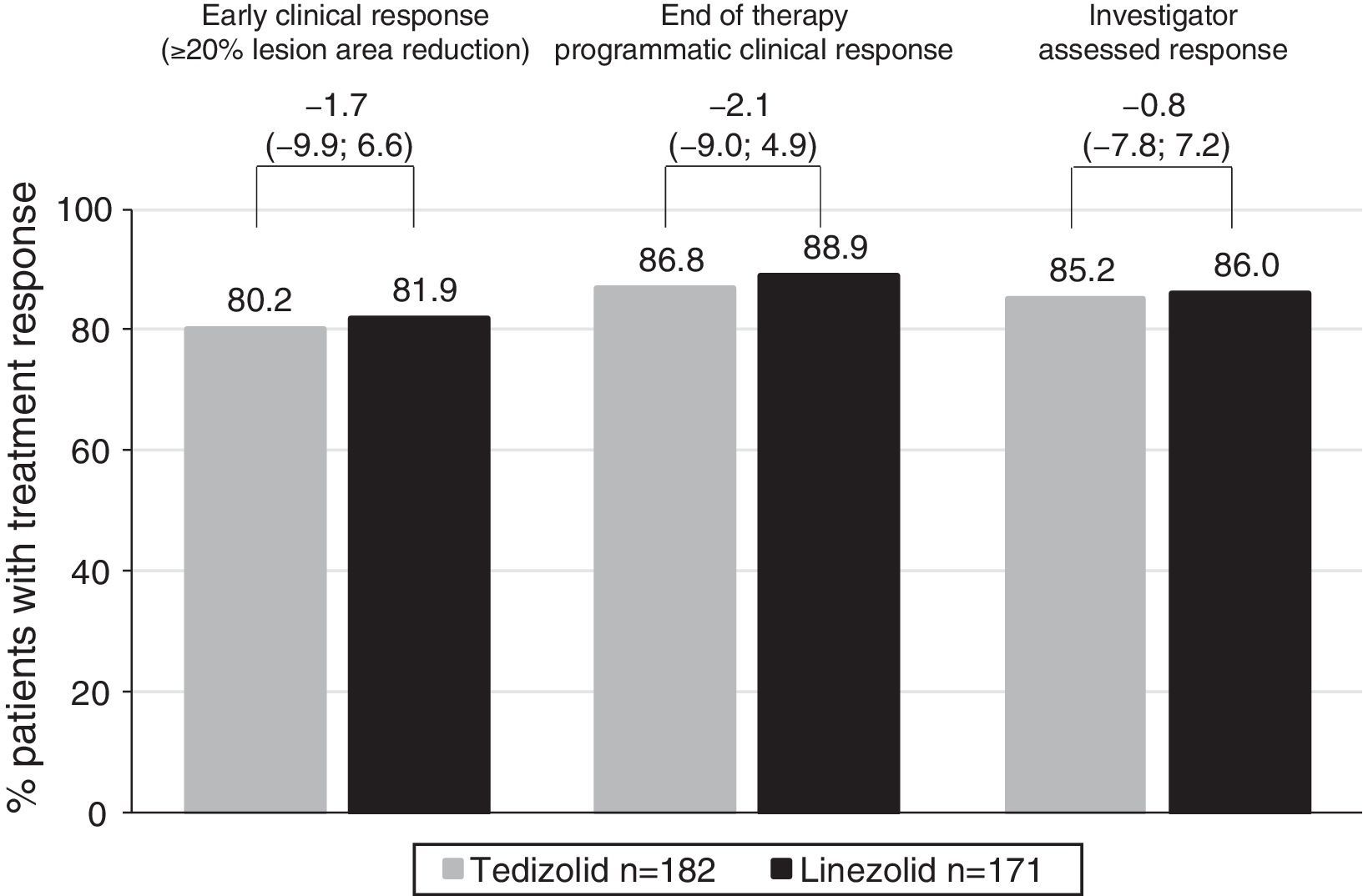
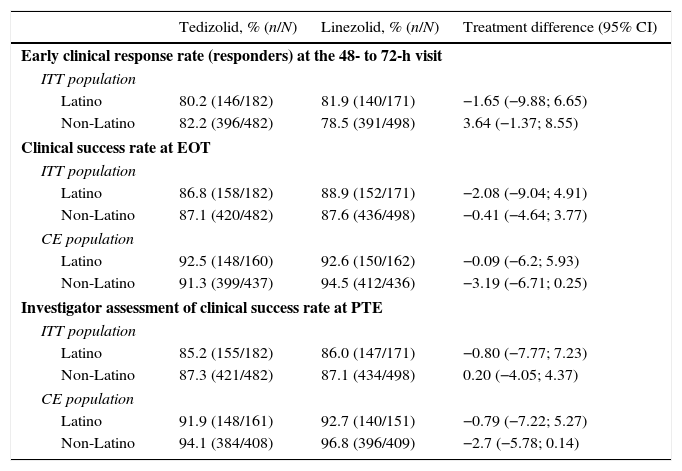
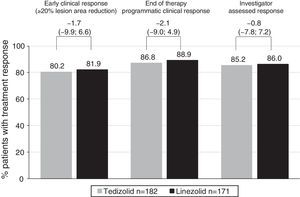
Efficacy of tedizolid and linezolid in Latino patientsThe efficacy of TZD was compared with LZD in the ITT population. In patients with Latino ethnicity in the integrated data of the ESTABLISH studies, comparable efficacy results were demonstrated between six days of TZD and 10 days of LZD (Fig. 1). Similarly to the overall population, in Latino patients tedizolid phosphate treatment demonstrated comparable efficacy with LZD treatment at the 48- to 72-h visit in the ITT population (TZD: 80.2% vs LZD: 81.9%; point difference: −1.65; 95% confidence interval (CI): −9.88; 6.65) (Table 2, Fig. 1).
Clinical efficacy of tedizolid vs linezolid in Latino and non-Latino patients.
| Tedizolid, % (n/N) | Linezolid, % (n/N) | Treatment difference (95% CI) | |
|---|---|---|---|
| Early clinical response rate (responders) at the 48- to 72-h visit | |||
| ITT population | |||
| Latino | 80.2 (146/182) | 81.9 (140/171) | −1.65 (−9.88; 6.65) |
| Non-Latino | 82.2 (396/482) | 78.5 (391/498) | 3.64 (−1.37; 8.55) |
| Clinical success rate at EOT | |||
| ITT population | |||
| Latino | 86.8 (158/182) | 88.9 (152/171) | −2.08 (−9.04; 4.91) |
| Non-Latino | 87.1 (420/482) | 87.6 (436/498) | −0.41 (−4.64; 3.77) |
| CE population | |||
| Latino | 92.5 (148/160) | 92.6 (150/162) | −0.09 (−6.2; 5.93) |
| Non-Latino | 91.3 (399/437) | 94.5 (412/436) | −3.19 (−6.71; 0.25) |
| Investigator assessment of clinical success rate at PTE | |||
| ITT population | |||
| Latino | 85.2 (155/182) | 86.0 (147/171) | −0.80 (−7.77; 7.23) |
| Non-Latino | 87.3 (421/482) | 87.1 (434/498) | 0.20 (−4.05; 4.37) |
| CE population | |||
| Latino | 91.9 (148/161) | 92.7 (140/151) | −0.79 (−7.22; 5.27) |
| Non-Latino | 94.1 (384/408) | 96.8 (396/409) | −2.7 (−5.78; 0.14) |
CE, clinically evaluable; CI, confidence interval; EOT, end-of-therapy; ITT, intent-to-treat; PTE, post-treatment evaluation.
At EOT, similar clinical success rates were found in the ITT (86.8% in TZD vs 88.9% in LZD; point difference: −2.08; 95% CI: −9.04; 4.91) and CE (92.5% in TZD vs 92.6% in LZD; point difference: −0.1; 95% CI: −6.2; 5.93) populations (Table 2). Furthermore, investigator-assessed clinical success rates were comparable in patients treated with TZD vs LZD at the PTE visit in the ITT and CE populations (Table 2).
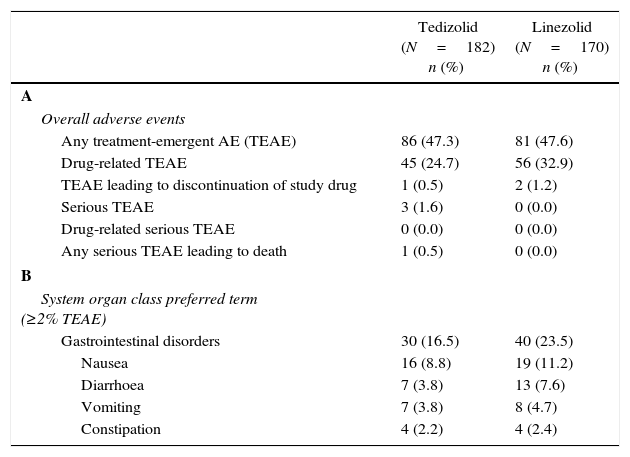
Safety and tolerability of tedizolid and linezolid in Latino patientsAmong Latino patients in the safety analysis population, 182 TZD-treated patients and 170 LZD-treated patients received at least one dose of the study drug and were eligible for safety analysis. In the Latino population, overall treatment-emergent adverse events (TEAE) rates were comparable in both TZD- (47.3%) and LZD- (47.6%) treated patients (Table 3A). The investigator-assessed rate of drug-related AEs was lower in the TZD treatment arm (45/182 patients, 24.7%) compared with the LZD treatment arm (56/170 patients, 32.9%), respectively. These results are similar to the overall integrated safety population (TZD: 148/662 patients [22.4%] vs LZD: 185/662 patients [27.9%], respectively).
Incidence of adverse events in the safety population of Latino patients.a
| Tedizolid (N=182) n (%) | Linezolid (N=170) n (%) | |
|---|---|---|
| A | ||
| Overall adverse events | ||
| Any treatment-emergent AE (TEAE) | 86 (47.3) | 81 (47.6) |
| Drug-related TEAE | 45 (24.7) | 56 (32.9) |
| TEAE leading to discontinuation of study drug | 1 (0.5) | 2 (1.2) |
| Serious TEAE | 3 (1.6) | 0 (0.0) |
| Drug-related serious TEAE | 0 (0.0) | 0 (0.0) |
| Any serious TEAE leading to death | 1 (0.5) | 0 (0.0) |
| B | ||
| System organ class preferred term (≥2% TEAE) | ||
| Gastrointestinal disorders | 30 (16.5) | 40 (23.5) |
| Nausea | 16 (8.8) | 19 (11.2) |
| Diarrhoea | 7 (3.8) | 13 (7.6) |
| Vomiting | 7 (3.8) | 8 (4.7) |
| Constipation | 4 (2.2) | 4 (2.4) |
AE, adverse event; TEAE, treatment-emergent adverse events.
Similar to the overall population, in Latino patients the most commonly reported AEs were GI related; however, the incidence of GI-related AEs was lower in TZD-treated patients compared with LZD-treated patients (16.5% vs 23.5%, respectively). Among GI adverse events, lower incidences of nausea, diarrhoea, vomiting were reported in TZD-treated Latino patients compared with LZD-treated Latino patients (Table 3B).
Rates of TEAEs leading to discontinuation of study drug were low and similar with TZD (0.5%) and LZD (1.2%) treatments in Latino patients (Table 3A). In this analysis, rates of serious TEAEs were also similar between the two treatment groups (TZD: 3 out of 182 patients [1.6%] and LZD: 0 out of 170 patients [0%], respectively) (Table 3A). No serious drug-related TEAEs occurred with either TZD or LZD, and only one TEAE leading to death was observed (in the TZD group) which was unrelated to study drug (Table 3A).
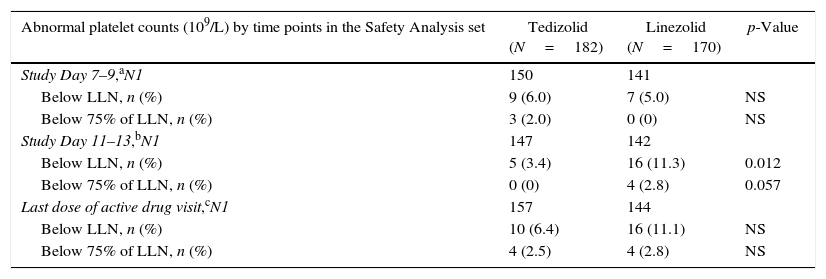
Laboratory resultsPlateletsAmong Latino patients, the proportion of patients with reduced platelet counts was similar between the two treatment arms at Day 7–9. Abnormal platelet count (i.e. below lower limit of normal [LLN]) was observed at approximately three-fold higher incidence in patients treated with LZD (11.3%) than TZD (3.4%) at Day 11–13 (Table 4); similarly, a higher incidence of abnormal platelet count was seen with LZD (11.1%) than TZD (6.4%) after the last dose of the active study drug, respectively.
Abnormal platelet counts (109/L) by timepoint in Latino patients.
| Abnormal platelet counts (109/L) by time points in the Safety Analysis set | Tedizolid (N=182) | Linezolid (N=170) | p-Value |
|---|---|---|---|
| Study Day 7–9,aN1 | 150 | 141 | |
| Below LLN, n (%) | 9 (6.0) | 7 (5.0) | NS |
| Below 75% of LLN, n (%) | 3 (2.0) | 0 (0) | NS |
| Study Day 11–13,bN1 | 147 | 142 | |
| Below LLN, n (%) | 5 (3.4) | 16 (11.3) | 0.012 |
| Below 75% of LLN, n (%) | 0 (0) | 4 (2.8) | 0.057 |
| Last dose of active drug visit,cN1 | 157 | 144 | |
| Below LLN, n (%) | 10 (6.4) | 16 (11.1) | NS |
| Below 75% of LLN, n (%) | 4 (2.5) | 4 (2.8) | NS |
LLN, lower limit of normal; NS, not significant; N1, number of patients in safety analysis population with non-missing data at baseline and the summarised visit.
The proportion of patients with substantially abnormal platelet count was very low in both treatment arms at all time points indicating the lack of clinically significant thrombocytopenia (Table 4).
Other laboratory resultsNo clinically meaningful changes were observed in haemoglobin, absolute neutrophil or leucocyte counts in either the TZD or LZD treatment arms in Latino patients (data not shown).
Population PK resultsIn the population PK analysis, no clinically meaningful covariate effects on TZD PK were found, although the final model did include the statistically significant covariates IBW and total bilirubin. Based on the model, summary measures of TZD exposure parameters were generated for Latino patients and compared with those of non-Latino patients.
In the population used for the PK modelling, there were no clinically meaningful differences between Latino (N=182) and non-Latino (N=482) ITT populations with regard to baseline demographic or laboratory parameters. Among the Latino patients, there were more males (73.1% vs 61.4%, respectively) and patients aged ≤65 years (95.1% vs 86.9%, respectively) compared with non-Latino patients. Latino patients had a slightly higher, but not statistically significantly, BMI (29.7±6.9kg/m2) than non-Latino patients (27.7±6.6kg/m2).
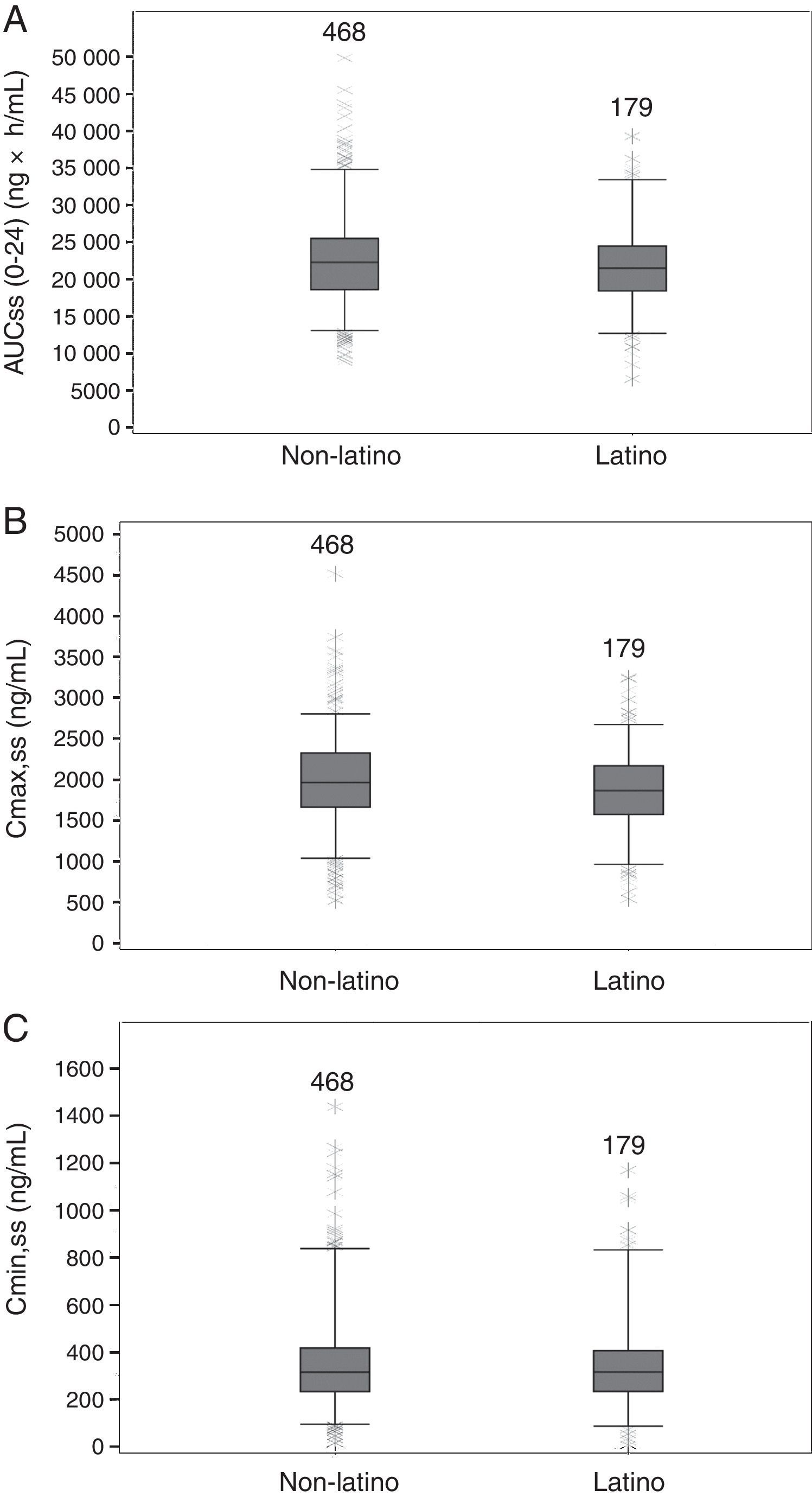
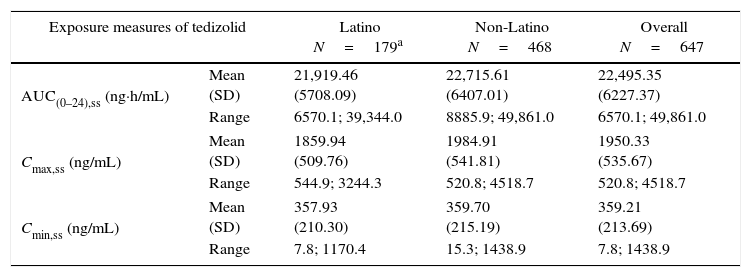
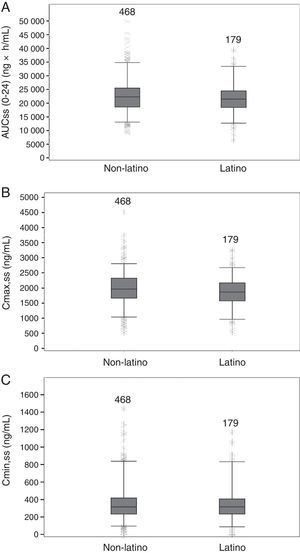
PK parameters expressing TZD exposure in Latino patients (N=179) were compared with those of non-Latino patients (N=468). There were no apparent differences at steady state between Latino and non-Latino patients (Table 5) in TZD AUC0–24h (21,919.5±5708.1ng×h/mL vs 22,715.6±6407.0ng×h/mL, respectively; Fig. 2A), Cmax (1859.9±509.8ng/mL vs 1984.9±541.8ng/mL, respectively; Fig. 2B) and Cmin (357.9±210.3ng/mL vs 359.7±215.2ng/mL, respectively; Fig. 2C).
Summary statistics of tedizolid exposure measures from Latino and non-Latino patients.
| Exposure measures of tedizolid | Latino N=179a | Non-Latino N=468 | Overall N=647 | |
|---|---|---|---|---|
| AUC(0–24),ss (ng·h/mL) | Mean (SD) | 21,919.46 (5708.09) | 22,715.61 (6407.01) | 22,495.35 (6227.37) |
| Range | 6570.1; 39,344.0 | 8885.9; 49,861.0 | 6570.1; 49,861.0 | |
| Cmax,ss (ng/mL) | Mean (SD) | 1859.94 (509.76) | 1984.91 (541.81) | 1950.33 (535.67) |
| Range | 544.9; 3244.3 | 520.8; 4518.7 | 520.8; 4518.7 | |
| Cmin,ss (ng/mL) | Mean (SD) | 357.93 (210.30) | 359.70 (215.19) | 359.21 (213.69) |
| Range | 7.8; 1170.4 | 15.3; 1438.9 | 7.8; 1438.9 | |
AUC(0–24),ss, area under the concentration curve at steady state; Cmax,ss, maximum concentration at steady state; Cmin,ss, minimum concentration at steady state; SD, standard deviation.
Boxplots of tedizolid AUC0–24, Cmax, Cmin at steady-state in Latino and non-Latino patients. Boxes are 25th, 50th, and 75th percentiles with median as horizontal line; whiskers are 5th and 95th percentiles. Asterisks show data points outside of this range. The number of subjects is provided above each box. (A) AUC(0–24),ss area under the concentration curve at steady state. (B) Cmax,ss, maximum concentration at steady state. (C) Cmin,ss, minimum concentration at steady state.
This post-hoc analysis of the integrated dataset of ESTABLISH-1 and ESTABLISH-2 was performed to compare the efficacy of tedizolid phosphate 200mg once daily IV/PO 6-day treatment with that of LZD 600mg twice daily IV/PO 10-day treatment in Latino patients with ABSSSIs. Furthermore, this analysis aimed to assess the safety profile of TZD in Latino patients and to compare estimates of TZD exposure in Latino and non-Latino patients. Based on the primary endpoint criteria, the results of the analysis showed comparable early clinical response rates with TZD and LZD treatments in Latino patients at the 48- to 72-h visit. Similar proportions of patients in the two treatment arms had sustained clinical success at the EOT and PTE visits. TZD was well tolerated in Latino patients with a lower incidence of GI AEs compared with LZD, similar to that seen with TZD in both non-Latino patients and the overall population. The incidence of thrombocytopenia was also lower for TZD than for LZD at Day 11–13, though the incidences were similar in the two groups at Day 7–9 and after the last dose of the active drug.
These results are in accordance with those in the overall integrated ITT population of ESTABLISH-1 and ESTABLISH-2.26–28,34 In the overall ITT population at the 48- to 72-h visit, approximately 80% of TZD- and LZD-treated patients demonstrated at least a 20% reduction in lesion size. A significant advantage was also seen with TZD treatment with regard to GI AEs28 and haematological changes in the overall population.26–28,34 Although similar trends were observed in the Latino subpopulation, significance was not demonstrated which was probably due to the lower number of patients in each arm. Nevertheless, the lower incidence of GI AEs and the lower risk of thrombocytopenia are favourable safety aspects of TZD treatment which might also apply to Latino patients. The improved haematological effects of TZD vs LZD may be the result of a smaller impact on mitochondria due to lower overall drug exposure and trough concentration.35
It is known that LZD treatment for more than 28 days is associated with neurotoxicity.36,37 A 6-month daily LZD exposure in rats, at 80mg/kg/day dose which reproduces the human daily exposure of 600mg BID, resulted in peripheral (sciatic) neuropathy within three months and optic nerve neuropathy within six months.37 On the contrary, rats exposed to daily administration of TZD for nine months, at a dose providing 8-fold higher exposure than the approved therapeutic dose, showed no signs of neurotoxicity during this time period.38 The lower risk of toxicity was also demonstrated in the ESTABLISH studies by the significantly lower incidence of GI AEs during the first six days of therapy when both drugs were administered, and not only over the entire observation period.26–28 No unexpected safety signal was observed in the Latino subpopulation or the full study safety population. TZD was well tolerated in both populations with similar incidences of TEAEs and reduced rate of drug-related AEs compared with those Latino patients who were treated with LZD.
LZD is an approved oral anti-MRSA drug in Latin American countries with the advantages of 100% oral bioavailability and no interethnic differences in PK properties.29 Previous Phase 1 studies have demonstrated high oral bioavailability of tedizolid (>90%).23 The population PK analyses revealed that there was no difference between Latino and non-Latino patients in the estimates of TZD exposure measures. The demographic parameters and the population PK model indicated that there were no clinically significant covariates, including race33; this suggests that the 200mg dose of tedizolid phosphate was appropriate for Latino patients for the treatment of ABSSSI and no dose adjustment is necessary for patients of this ethnicity. Previously, no statistical influence of sex, age, race, ethnicity, body weight, BMI, ALT, AST, or creatinine clearance was found on TZD population PK parameters.33 IBW and total bilirubin were found to be statistically significant covariates for observed variability of PK parameters; however, neither of these effects were determined to be clinically relevant.33
As endorsed by current antimicrobial stewardship recommendations, shorter courses of treatment are advocated in order to minimise the risk of antimicrobial resistance development, optimise the safety profile and improve patient compliance with treatment. TZD demonstrates at least 4-times greater potency than LZD against a wide range of Gram-positive pathogens, including MRSA and VRE.21,22 The greater potency was the result of modification of the core structure in three different ways.20 These modifications and the greater potency led to the hypothesis that TZD may be efficacious at a lower dose and in shorter treatment duration compared with LZD. This hypothesis was demonstrated in a Phase 2 study where the lowest dose tested (200mg QD) proved as efficacious as higher doses in patients with ABSSSI,39 and later confirmed in the two pivotal Phase 3 studies. Based on these results, tedizolid phosphate is now approved at a dose of 200mg as a once daily IV and/or oral therapy for the management of patients with ABSSSIs in the US,40 Europe, Canada, Singapore, Kuwait, and Chile, with a short treatment duration of six days.40
High bacteriological eradication rate and consequently high clinical cure rate were demonstrated in patients at the PTE visit in the overall population. Susceptibility testing to TZD and LZD showed that all MRSA strains isolated in the trials were susceptible to TZD according to the current FDA and EU breakpoints [Susceptible: ≤0.5μg/mL].26–28 Bacteriological results were not obtained for Latino patients; however, it is expected that TZD would be efficacious against Gram-positive pathogens causing infection in Latino patients enrolled into the studies. A recent surveillance study of nearly 7000 Gram-positive isolates (including approximately 4500 S. aureus isolates) conducted globally demonstrated a high rate of susceptibility to TZD and LZD with only 16 isolates (including seven S. aureus, five S. epidermidis, and four enterococci) being resistant to LZD.22 However, some of these isolates remained susceptible to TZD if they carried a plasmid encoding for Cfr methylase only.22
One of the limitations of this analysis was that it was a post-hoc subgroup analysis with lower patient numbers per treatment group; therefore, the analysis was not powered to demonstrate statistical difference between Latino and non-Latino populations in efficacy and safety parameters. Consequently, further studies are required to strengthen the clinical value of the efficacy and safety findings and results of the current analysis. An ongoing global Phase 3 clinical study which compares the efficacy and safety of TZD and LZD in patients with nosocomial pneumonia will provide additional information regarding the safety of TZD in Latino patients.
Ethnic factors have been shown to influence the safety and efficacy of several compounds; in fact, the FDA and EMA have encouraged the presence of patients from distinct ethnicity and/or race in clinical trials of drugs under development.30,31 It is hypothesised that inter-ethnic differences in the PK of antibiotics depend on the first-pass metabolism and transport mechanisms because some agents demonstrate relatively low bioavailability.29 These inter-ethnic differences appear to be common, although their clinical relevance must be investigated.29 The fact that the pharmacokinetic/pharmacodynamic properties of TZD were not affected by ethnicity (or race) might explain the similar findings in efficacy and safety of TZD between Latino and non-Latino patients enrolled in the ESTABLISH trials.
ConclusionIn conclusion, the results of the current analysis suggest that TZD was efficacious and well tolerated in the management of Latino patients with ABSSSIs. The population PK analysis of patients enrolled into ESTABLISH-1 and ETABLISH-2 suggest that no dose adjustment is needed for Latino patients for the treatment of ABSSSI. Furthermore, these findings indicate that TZD may be an appropriate choice of treatment for Latino patients with ABSSSIs who are either hospitalised or outpatients due to the high potency and high oral bioavailability of the agent; this is also supported by the favourable safety profile of TZD demonstrated in the current study. However, prescription of TZD (or linezolid) for Latino patients should be considered according to the approved Summary of Product Characteristics and local guidelines.
FundingFunding for the ESTABLISH-1 and ESTABLISH-2 clinical trials was provided by Merck & Co., Inc., Kenilworth, NJ, USA. The post-hoc analysis of the Latino subgroup was jointly funded by Merck & Co., Inc., Kenilworth, NJ, USA and Bayer HealthCare, Germany.
Conflicts of interestSF and PP are current employees of Merck & Co., Inc., Kenilworth, NJ, USA. EF is a former employee of Merck & Co., Inc., Kenilworth, NJ, USA. XZ is an employee of Bayer Healthcare, Beijing, China. JFC-M is an employee of Bayer HealthCare, Mexico. TT is an employee of Bayer Yakuhin, Osaka, Japan. JP and JF-K are employees of Cognigen Corporation, a subsidiary of Simulations Plus, Inc., Buffalo, NY, USA. AO participated as a clinical investigator for Bayer HealthCare, received speaker honoraria from Bayer and has been clinical investigator for Astellas Pharma, MSD, and Sangui labs. ECN has received grant support from Cubist and Novartis, honoraria as speaker from AstraZeneca, Pfizer and Merck and as consultant from Cerexa, and has recently participated as investigator in trials of AstraZeneca, Trius and Cempra.
Both the ESTABLISH-1 and -2 clinical trials were sponsored by Merck & Co., Inc., Kenilworth, NJ, USA. The analysis of the Latino subgroup was jointly supported by Merck & Co., Inc., Kenilworth, NJ, USA and Bayer HealthCare, Germany. Editorial support was provided by Highfield Communications, Oxford, United Kingdom, sponsored by Bayer HealthCare, Germany.