To explore the distribution and clinical manifestations of rhinovirus infection in wheezing children, and compare the clinical differences between rhinovirus- and respiratory syncytial virus-induced wheezing.
Materials and methodsThis prospective cohort study was carried out in Children's Hospital of Soochow University from Dec 2012 to Nov 2014. We enrolled consecutive hospitalized children <60 months of age presented with wheezing. Clinical data including cough, fever, dyspnea, crackles were recorded by pediatricians on the first day of admission. Meanwhile, nasopharyngeal aspirates were obtained to test for respiratory viruses, by using polymerase chain reaction method for rhinovirus, human bocavirus, and human metapneumovirus, and direct immunofluorescence assay to test for respiratory syncytial virus, adenovirus, parainfluenza virus types 1–3, and influenza virus types A and B.
ResultsRhinovirus was a main causative agent isolated in 14.7% of the hospitalized wheezing children in Suzhou, China, being second to respiratory syncytial virus (21.0%). Different from respiratory syncytial virus infection, which peaked in winter months, rhinovirus could be detected all year round, peaked between July and September, and in November. Children with rhinovirus infection were older and presented with more often allergic sensitizations, blood eosinophilia, and leukocytosis than those of respiratory syncytial virus infection. Logistic regression analysis revealed that rhinovirus-infected children experienced earlier wheezing more often than respiratory syncytial virus children (odds ratio, 3.441; 95% confidence interval, 1.187–9.979; p=0.023).
ConclusionRhinovirus was a main viral pathogen in wheezing children, especially in summer time. Rhinovirus-induced wheezing was different from respiratory syncytial virus, apart from seasonal epidemics; these two groups differed with regard to age, allergic sensitizations, laboratory test, and history of wheezing episodes.
A range of respiratory viruses is known to cause acute wheeze, including bronchiolitis and asthma exacerbations. The most frequently reported viral pathogen has been respiratory syncytial virus (RSV) in children less than two years. Other viruses included adenovirus, influenza virus (FLU), parainfluenza virus (Pinf), rhinovirus (RV), human metapneumovirus (hMPV), adenovirus (ADV), and human bocavirus (HBoV). RV caused a wide range of respiratory diseases ranging from common cold to life-threatening pneumonia, and was popular as a major cause of common colds. By the age of two years, about 91.3% of the children once had RV infection.1
Over the past years, the role of RV in wheezing patients might have been underestimated since the detecting technology for RV was less sensitive. From 2006, with the development of PCR methods, RV was recognized as an important cause of lower respiratory infections in young children, with a detection rate ranging from 5% to 40%.1–6 In addition, RV could trigger acute wheezing and was highly prevalent in asthma exacerbations.7 Moreover, childhood RV induced wheezing may be more closely related to adult asthma than RSV.8,9
The clinical association of wheezing illness with RV has not been well described in China. Herein, we conducted a prospective cohort study of virus induced wheezing in children, aimed to analyze the distribution and clinical manifestations of RV and compare with RSV-induced wheezing.
Materials and methodsPatient enrollment and data collectionWe conducted a prospective cohort study at the Children's Hospital of Soochow University from Dec 2012 to Nov 2014. This study was approved by the Institutional Review Board of Children's Hospital of Soochow University. A standardized protocol was used to enroll a target number of consecutive patients from the inpatient wards of pulmonology. Children aged <60 months who presented with wheezing were included. The exclusion criteria were preterm birth ≤37 weeks of gestation, history of a diagnosis of chronic lung disease, congenital heart disease, and immunodeficiency. After obtaining informed consent from the parents, clinical–epidemiological information was collected by pediatricians on admission. Nasopharyngeal samples were taken within 24h of admission by trained researchers.
Laboratory analysisRT-PCR methods were applied to test RNA of RV and hMPV, and DNA of HBoV. Direct immunofluorescence assay was performed on nasal aspirates by using murine monoclonal antibodies (Chemicon) to test RSV, FLU A, FLU B, and Pinf 1, 2, 3.
Statistical analysisAll of the statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS; version 19.0). Categorical variables were compared using chi-square test. Multivariate analysis was performed using logistic regression to determine odds ratios (OR). p-Value <0.05 was considered statistically significant.
ResultsStudy cohortSeven hundred and fourteen patients were enrolled in the study. Five enrolled patients were excluded because of incomplete data, leaving a total of 709 subjects in the study cohort. Enrolled and non-enrolled children were similar in age and gender (both p>0.05). Among the 709 children, 277 children were under six months old, 335 aged 6–24 month, and 97 aged 24–60 months. The male to female ratio was 2.4.
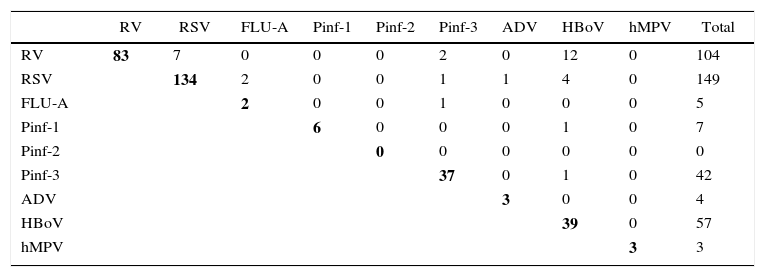
Distribution of respiratory viruses in wheezing childrenAmong the 709 wheezing children, 371 (52.3%) were positive for any of the viruses tested. In order of frequency, RSV (21.0%) and RV (14.7%) were the most frequently detected viruses, followed by HBoV (8.0%), Pinf-3 (6.1%), Pinf-1 (1.0%), FLU-A (0.7%), ADV (0.6%), and hMPV (0.4%). Co-infections were observed in 32 (4.5%) patients, with RV as the most frequently recovered pathogen accounting for 21 (65.6%) patients of co-infections, most of which were combinations of RV with HBoV. No triple infection was found. The result is shown in Table 1.
Distribution of respiratory viruses in wheezing children.
| RV | RSV | FLU-A | Pinf-1 | Pinf-2 | Pinf-3 | ADV | HBoV | hMPV | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| RV | 83 | 7 | 0 | 0 | 0 | 2 | 0 | 12 | 0 | 104 |
| RSV | 134 | 2 | 0 | 0 | 1 | 1 | 4 | 0 | 149 | |
| FLU-A | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 5 | ||
| Pinf-1 | 6 | 0 | 0 | 0 | 1 | 0 | 7 | |||
| Pinf-2 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| Pinf-3 | 37 | 0 | 1 | 0 | 42 | |||||
| ADV | 3 | 0 | 0 | 4 | ||||||
| HBoV | 39 | 0 | 57 | |||||||
| hMPV | 3 | 3 |
Number of samples containing each set of viruses, in boldface, simple infections, no influenza virus B was detected. RV, rhinovirus; RSV, respiratory syncytial virus; FLU, influenza virus; Pinf, parainfluenza virus; ADV, adenovirus; hMPV, human metapneumovirus; HBoV, human bocavirus.
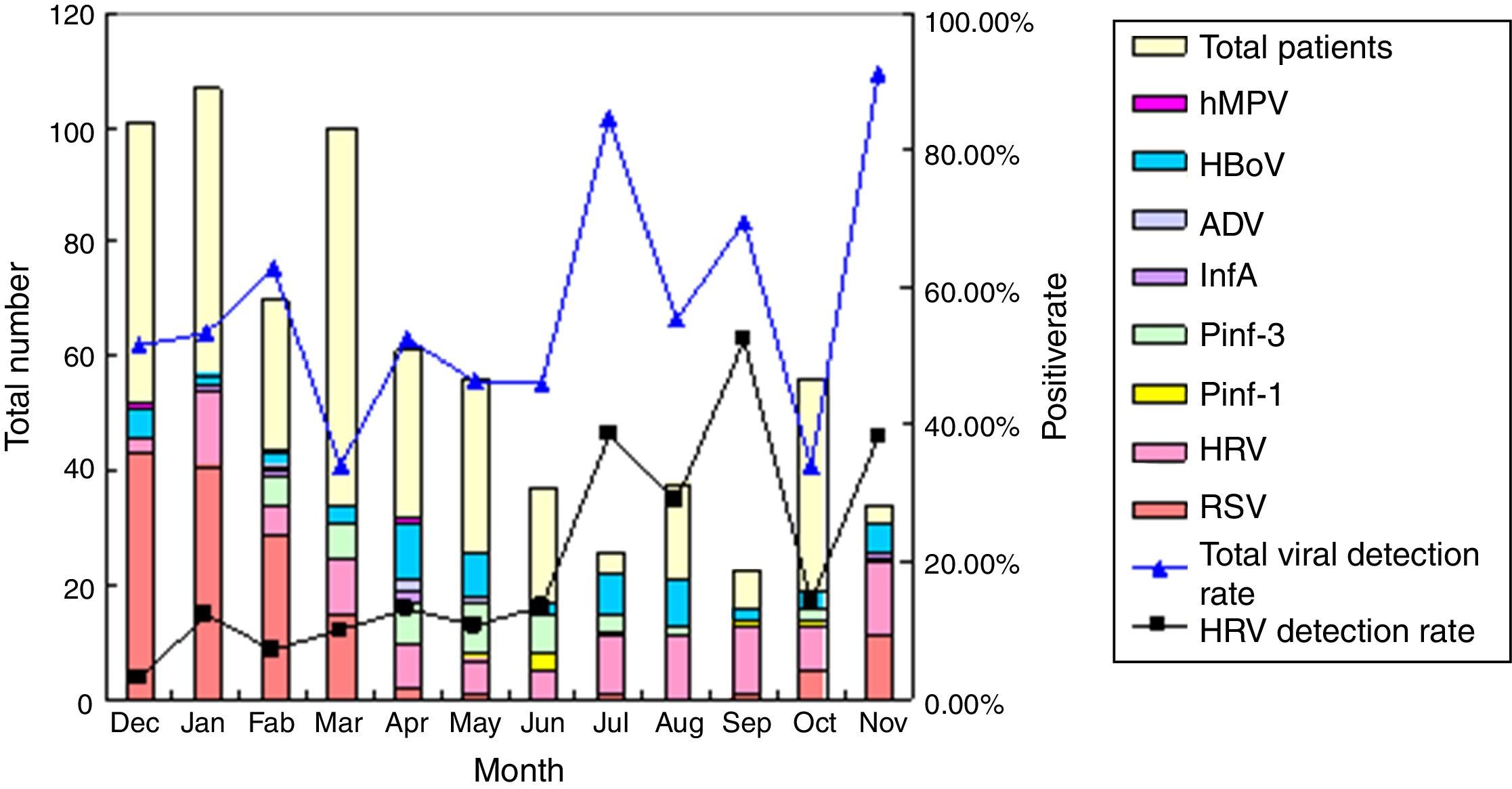
RV infection could be detected throughout the year with 23% identified in spring (Mar–May), 25% in summer (Jun–Aug), 32% in autumn (Sep–Nov), and 20% in winter (Dec–Feb). The majority of RV cases occurred from July to September and in November. The detection rate of RV peaked in November (38.24%). On the contrast, most RSV infections peaked in winter months (73.8%), which might lead to increased hospitalization. Although hospitalization of wheezing patients was less common in warm months, the total viral detection rate was relatively higher, and the most frequently detected viruses were RV and HBoV. The fluctuation of total viral detection rate throughout the year was in keeping with that with RV detection rate (Fig. 1). ADV, FLU A and hMPV were occasionally found.
Demographic and clinical characteristicsOf the RV isolated, 25 cases were <6 months (positivity rate, 9.03%), 29 cases were 6–12 months (14.22%), 39 were 12–36 months (23.35%), 10 were 36–60 months (27.03%), and one was >60 months (4.17%). Children aged between 36 and 60 months had the highest infection rate, but the data was not statistically significant different. Of the RSV isolated, 88 cases were <6 months, 40 cases were 6–12 months, 18 were 12–36 months, and three were 36–60 months. Children aged under 6 months got the highest RSV infection rate (p<0.001) and the median age of children with RV infection were higher than those with RSV infection (<0.001).
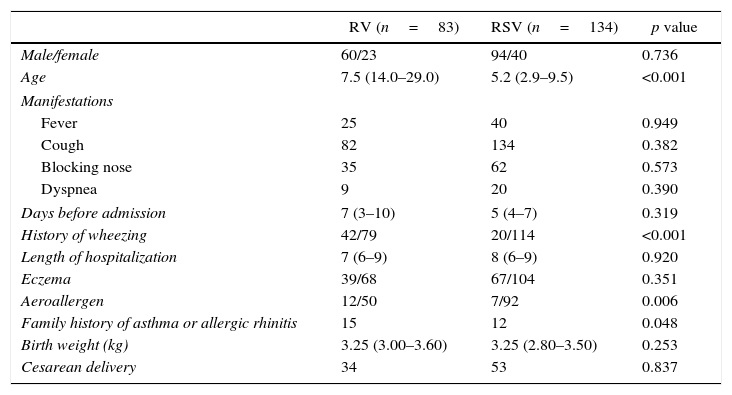
Since RSV and RV were the main pathogens isolated in wheezing illness, single RV and single RSV infection were compared and analyzed. Table 2 depicts the demographic and clinical characteristics of RV and RSV. Fever, cough, dyspnea, hospital stay did not differ between the two groups. However, children with RV infection had more often experienced earlier wheezing than RSV infection. In addition, allergic sensitizations were more common in children with RV infection.
Demographic characteristics, medical history, and clinical course of wheezing children with RV and RSV infection.
| RV (n=83) | RSV (n=134) | p value | |
|---|---|---|---|
| Male/female | 60/23 | 94/40 | 0.736 |
| Age | 7.5 (14.0–29.0) | 5.2 (2.9–9.5) | <0.001 |
| Manifestations | |||
| Fever | 25 | 40 | 0.949 |
| Cough | 82 | 134 | 0.382 |
| Blocking nose | 35 | 62 | 0.573 |
| Dyspnea | 9 | 20 | 0.390 |
| Days before admission | 7 (3–10) | 5 (4–7) | 0.319 |
| History of wheezing | 42/79 | 20/114 | <0.001 |
| Length of hospitalization | 7 (6–9) | 8 (6–9) | 0.920 |
| Eczema | 39/68 | 67/104 | 0.351 |
| Aeroallergen | 12/50 | 7/92 | 0.006 |
| Family history of asthma or allergic rhinitis | 15 | 12 | 0.048 |
| Birth weight (kg) | 3.25 (3.00–3.60) | 3.25 (2.80–3.50) | 0.253 |
| Cesarean delivery | 34 | 53 | 0.837 |
Regarding laboratory tests, leukocytosis (>10×109/L) was present in 51 of the 83 RV-infected cases (61.44%), higher than in those with RSV infection (37.31%, p=0.001), eosinophilia was noted in nine of the 83 RV-infected cases (10.84%), higher than in those with RSV infection (1.49%, p=0.004). Elevated CRP (>15mg/L) was observed in eight (9.64%) RV-infected cases, and in five (3.73%) RSV-infected patients (p=0.075).
As assessed by multivariate logistic regression analysis including age, history of wheezing, aeroallergen, family history in the model, RV infection was independently associated with a higher risk of earlier wheezing episodes (OR, 3.441; 95% confidence interval [CI], 1.187–9.979; p=0.023); eosinophilia and leukocytosis were more often observed in children with RV infection (OR, 11.584, 95% CI, 1.080–124.224; p=0.043, OR, 1.188, 95% CI, 1.070–1.320; p=0.001).
DiscussionIn Suzhou, one or more viruses could be identified in half of the wheezing children. As expected, RSV was the main cause of wheezing illness. RV was identified in a considerable number of patients (104 of 709, 14.7%), a finding comparable with the report of Miller.10 RV could be detected all year round, peaked in November as reported in Changsha, China.11 The epidemiology of RV varied by areas and season of year.12 Some studies showed no seasonality,2,9 whereas others found RV peaking in spring and autumn,10,13 which might have been related to different rhinoviral species in different regions and seasons. On the other hand, this one-year study might be too short to conclude about seasonality. In contrast, RSV presented with a seasonal pattern, predominantly in the winter months, which contributed to an increase in hospitalization during the winter.
As shown by Monto,12 in summer (July and August) the overall number of wheezing patients was lower but respiratory viral detection was higher, which might have been a result of an increasing rate of RV infection at this time of year, indicating that RV was the main viral pathogen in wheezing patients at summer time.
Previous studies showed that co-infections with other respiratory viruses are more common in RV-infected patients, found in 20–40% of.14,15 In agreement with these, 21 patients (20.2%) infected with RV were co-infected with a second virus, which might be related to the persistence of RV in airways. Previous studies found that RV could persist in airways for up to two months,2,16 leading to an “over detection” of RV. In addition, some viruses could be detected in healthy children, including RV that could be detected in 10–25%.17–19 Thus, it is uncertain whether RV was pathogenic or not in these co-infections, while some studies have found that RV increased disease severity16,20 in contrast to others.2,17 As for this study, RV did not seem to aggravate the course of the respiratory disease (data not shown).
Clinical manifestations of RV like dyspnea, oxygen supplementary, fever, and hospitalization length were similar to those of RSV infection. RV seemed to be more associated with allergic sensitizations and blood eosinophilia. The interaction between allergy and viral infection is complex. On one hand, allergen may increase the expression level of intercellular adhesion molecule-1 (ICAM-1) on mucosal epithelial cells, which is the attachment molecule for the majority of RV serotypes, thus facilitating RV infection.21 On the other hand, respiratory viral infections including RV, may trigger allergic conditions. Using a longitudinal birth cohort, Jackson et al. demonstrated that allergic sensitization increased the risk of RV-associated wheezing and the converse was not true.22 Since there few reports on the subject, more research is needed to better elucidate the interactions between RV and allergy.
Leukocytosis may also occur in viral infections, as more than half of the RV-infected patients presented with leukocytosis, notably higher than that of RSV-infected patients. Previous studies had similar findings.2,19 Whether these RV infections were accompanied by bacterial infection was uncertain.
In multivariate analysis, a history of wheezing was a statistically significant factor that distinguished children with RV from those with RSV, as also reported by Miller et al.23 RV infection has been shown to be closely related with asthma exacerbations.24,25 There might be some explanations why children with a history of wheezing were more susceptible to RV infection1: patients with RV were older, i.e. had more opportunities to suffer from wheezing illnesses2; Some studies found that RV-induced wheezing in early life increased the risk of asthma3; Some studies speculated that children with a predisposition to asthma were more likely to be infected with RV. Among children who had experienced earlier wheezing some could be asthmatic and not be aware of it. Thus, is RV a risk factor for asthma or it merely unmasks the genetic predisposition to asthma? More research is warranted to dig into this phenomenon.
It should be noted that a major limitation of this study is that RSV diagnosis was performed by antigen detection method while RV diagnosis was by PCR, which might have underestimated the prevalence of RSV. Second, the phylogenetic analysis of RV was not performed, as the monthly distribution and clinical manifestations might differ among different RV species. Thus, more work is essential for a better understanding of the seasonal epidemiology of different RV species.
In conclusion, RV was a main cause of wheezing illness, especially in summer. RV might be more related to recurrent wheezing and allergic sensitizations compared with RSV infections. However, a larger study is needed to learn more about RV infection.
Conflicts of interestThe authors declare no conflicts of interest.