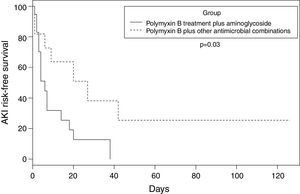
A retrospective cohort study, were evaluated: polymyxin B plus aminoglycosides or polymyxin B plus other antibiotics. Any degree of acute kidney injury occurred in 26 (86.6%) patients. The median time to acute kidney injury was 6.0 (95% CI 3–14) days in the polymyxin-aminoglycoside containing regimen group, against 27.0 (95% CI 6–42) days in the polymyxin with other antimicrobial combinations group (p=0.03). Polymyxin B with aminoglycosides group progressed faster to any degree of renal dysfunction.
Nephrotoxicity is an important adverse effect associated with polymyxin (polymyxin B or colistin)-based treatments, with rates of acute kidney injury (AKI) ranging from 20% to 60%. Recently, some studies have shown a better nephrotoxicity profile for polymyxin B compared to colistin.1–4
However, all studies that have evaluated the use of polymyxins for the treatment of infections had relatively short treatment periods, i.e., 7–14 days. No study thus far has addressed the incidence and evolution of AKI in patients treated for longer periods with polymyxins, specifically in conjunction with aminoglycosides or other antimicrobials.
Recently, we evaluated the clinical and epidemiological features of post-cardiac surgery patients with mediastinitis infected with carbapenem-resistant Enterobacteriacea (CRE).5 Due to the peculiarity of the infection, polymyxin B was the treatment of choice, and a prolonged period of four to six weeks was recommended.6
This study aimed to evaluate the real-world evidence for the development of AKI during prolonged periods of polymyxin B-based antimicrobial combinations assessed by specific criteria.7
Material and methodsThis was a retrospective cohort study conducted at a 350-bed hospital specialized in cardiology and cardiovascular surgery in São Paulo, Brazil between December 2010 and June 2014.
Thirty patients diagnosed with mediastinitis based on the CDC criteria,8 who were infected with CRE were included in the analysis of time to evolution to AKI.
Patient charts were reviewed to capture demographic and clinically relevant data, including comorbidities, baseline serum creatinine, weight, body mass index (BMI), and APACHE II score while at the intensive care unit (ICU). The outcomes measured were development of AKI during treatment, which was assessed according to the *RIFLEb criteria.7
All Gram-negative strains recovered from patients diagnosed with mediastinitis were submitted to the local microbiology laboratory for identification and determination of antimicrobial susceptibility profiles by VITEK® 2 (bioMérieux, Marcy-l’Étoile, France). Resistance was defined as a minimum inhibitory concentration (MIC) of ≥4g/mL for carbapenems (imipenem and meropenem), according to the Clinical and Laboratory Standards Institute (CLSI),9 and resistance to polymyxins was defined by a colistin MIC≥4g/mL.10,11 The screening test of carbapenemase was performed using a modified Hodge test as recommended by the CLSI,9 and the detection of carbapenemase genes (blaKPC, blaNDM, blaIMP, blaVIM, blaGES and blaOXA-48-like) was determined using real-time PCR12 for all isolates recovered.
Therapy management was performed at the discretion of the infectious diseases (ID) attending physician, and the present study considered the real-world evidence of the prescribed regimens. To categorize the antimicrobial combination into groups, we separated the combination regimens into two distinct risk groups: (a) regimens containing polymyxin B and aminoglycosides±other antimicrobials and (b) regimens containing polymyxin B±other antimicrobials (but without aminoglycosides).
The following intravenous antimicrobial doses were used at the institution for the treatment of CRE mediastinitis: polymyxin B 25,000UI/kg/day (no loading dose), amikacin 15mg/kg/day q24h, gentamicin 5mg/kg/day q24h, meropenem 1g q8h, imipenem 500mg q6h, tigecycline 100mg loading dose followed by 50mg q12h, and ciprofloxacin 400mg q12h.13
Each subject was included only once in the analysis. Descriptive statistics was used to describe the overall characteristics of the cohort. All 30 patients included in the two regimen groups were analyzed using the log rank test to estimate the time to evolution to AKI according to the RIFLE criteria.7 Categorical variables were compared using the Fisher or Chi-square test, and quantitative variables using t-Student or Mann–Whitney tests, as appropriate. p-Value ≤0.05 was considered statistically significant.
The statistical package SPSS 19 (IBM, New York, USA) was used for dataset management and analysis.
This study was approved by the Ethical Committee of Instituto Dante Pazzanese de Cardiologia.
Results and discussionThe most common surgical procedures performed were coronary artery bypass grafting (CABG) and/or valve replacement (70%). The demographic and clinical characteristics found in both treatment groups are reported in Table 1.
Demographic and clinical characteristics of patients with mediastinitis in both treatment groups.
| Patient demographics | Total n=30 | Polymyxin B plus other antimicrobials n=11 | Polymyxin B plus aminoglycoside/other n=19 | p-Value |
|---|---|---|---|---|
| Female sex,n(%) | 12 (40) | 4 (36.4) | 8 (42.1) | 1.00 |
| Race White,n(%) | 23 (76.7) | 8 (72.7) | 15 (79) | 1.00 |
| Body mass index (BMI) mean (SD) | 30 | 31.44 (8.10) | 30.41 (7.68) | 0.735 |
| BMI>30,n(%) | 12 (40) | 4 (36.3) | 8 (42.1) | 1.00 |
| APACHE Index (AI) when CRE treatment starts/No. patients (P) | 13 (P) (43.33%) | 12 (AI) 4 (P) | 15.78 (AI) 9 (P) | 0.414 |
| Creatinine (mg/dL) before surgery mean (SD) | 30 | 0.90 (0.30) | 1.11 (0.44) | 0.268 |
| ICU stay before CRE mediastinitis,n(%) | 14 (46.6%) | 4 (36.4%) | 10 (52.6%) | 0.466 |
| CRE mediastinitis | 30 | 11 | 19 | |
| Total Polymyxin Resistant (PR-CRE) | 12 | 2 (18.2%) | 10 (52.6%) | 0.121 |
| Treatment | ||||
| Polymyxin B, n (%) | 30 | 11 | 19 | |
| Length of treatment (days) | 30 (P) | 44 days | 25 days | 0.019 |
| Total doses/day IU | ||||
| Median | 1,500,000 | 1,500,000 | 1,500,000 | 0.215 |
| Minimal | 750,000 | 1,300,000 | 750,000 | |
| Maximum | 3,200,000 | 3,200,000 | 2,200,000 | |
| Aminoglycosides, n (%) | 19 (63.3) | – | 19 | |
| Length of treatment median mean range (days) | – | – | 20 (6–45) | |
| Patients any AKI degree – n | 26 | 9 | 17 | |
| End Stage Renal disease | 1 (9.1%) | 5 (26.3%) | 0.372 | |
| Mean time to AKI in days | – | 27 days | 6 days | 0.03 (CI 95% 3–14) |
| Complete renal function recovery after treatment (SCr<1.5mg/dL), n (%) | 9 (45) | 5 (62.5) | 4 (33) | 0.36 |
| In-hospital mortality | 11 (36.6%) | 1 (9.1%) | 10 (52.6%) | 0.023 |
CRE mediastinitis was most commonly due to Klebsiella pneumoniae (n=20), Enterobacter aerogenes (n=8), and Enterobacter cloacae (n=2); blaKPC was the only gene detected and present in all CRE strains. Among the 30 patients, three (10%) were treated with double antimicrobial therapy, and 90% were treated with triple, quadruple or even quintuple antimicrobials according to the clinical judgment of the severity and/or resistance of the CRE to polymyxin B.
Any degree of AKI occurred in 26 (86.6%) patients. Of these, 20 (77%) were classified as risk, injury or failure and six (23.1%) as end-stage renal disease. In the polymyxin B/aminoglycosides treatment group, the mean time to AKI was six days, compared to 27 days in the polymyxin B-other antimicrobials (without aminoglycosides) treatment group (p=0.03). Fig. 1 shows the estimate of AKI-free days survival risk stratified by the log-rank test between the treatment groups of polymyxin B-aminoglycoside and polymyxin B-other antimicrobials regimens.
Among the 20 patients who had no end-stage renal disease, 9/20 (45%) had creatinine ≤1.5mg/dL at the end of treatment, 4/12 (33.3%) in the polymyxin-aminoglycoside group and 5/8 (62.5%) in the polymyxin-other combination group (p=0.36).
Among the 12 polimixin-resistan-CRE patients (PR-CRE), 10 (83.3%) were in the group treated with polymyxin/aminoglycoside (p=0.121). Duration, assessed in days, of polymyxin treatment was significantly shorter in the group that used aminoglycosides compared to the polymyxin/other antimicrobials group (25×44 days, p=0.019), which was most likely due to progression to renal failure and the option by the clinician to reduce damage. Hospital mortality was higher in the polymyxin B/aminoglycoside group, p=0.023, which could have been due to the multifactorial risks involved, including more PR-CRE cases in this group. Although the difference in the number of PR-CRE cases was not significant, it was clearly more difficult to treat the infection and more aggressive procedures were used leading to loss of renal function with subsequent need for hemodialysis.
This unique case series highlights the difficulties in the treatment of CRE mediastinitis with prolonged use of polymyxin B-based combination regimens. Treating mediastinitis after cardiac surgery is rather challenging, which is even more difficult if CRE or PR-CRE are involved. The occurrence of PR-CRE in Brazil14 and worldwide15,16 is increasing and is becoming a public health concern given the dearth of viable therapeutic options available. The benefit of treatment with polymyxin combined with carbapenems for CRE infections has previously been reported,17–20 but in cases of PR-CRE, the number of clinical studies has been limited.15 Our case series is limited in its ability to determine the causes of renal dysfunction, but it is reasonable to assume that among the factors related to progression to renal dysfunction, the concomitant use of polymyxin B and aminoglycosides is an important factor due to higher propensity to cause any degree of AKI.
Regimens containing polymyxin B and aminoglycosides should be observed with caution, particularly in this patient population due to increased potential for nephrotoxicity, but in some cases, this regimen is the only treatment available for difficult to treat infections.
The main limitations of this single-center study were its sample size and the retrospective nature of the study design. Thus, definite conclusions are precluded due to low statistical power. Despite the limitations, limited data are available on polymyxin B and prolonged periods of treatments, and thus, our results provide useful clinical information to better understand extended periods of use of polymyxin B and other antimicrobial combinations with respect to AKI development.
FundingFundação de Amparo à Pesquisa do Estado de São Paulo - FAPESP – Auxílio à Pesquisa: 12108-3/2014
Conflicts of interestThe authors declare no conflicts of interest.
Celia Harumi Hiroshi – secretary.
João Italo França – LEE Laboratory of Epidemiology and Statistics – Instituto Dante Pazzanese de Cardiologia.
RIFLE: acronym of Risk, Injury, Failure, Loss and End-stage of kidney disease. Risk – serum creatinine (SCr) increased to 2–3 times the baseline, Injury – SCr increased to > 3 times the baseline, Failure – SCr ≥ 4mg/dL, Loss of function – persistent acute renal failure.7
This study was partially presented as a poster at that 55th ICAAC 2015, 17–21, 2015 San Diego, CA, and as an oral presentation (Abstract O-3) at the 2nd International Conference on Polymyxins 2015, September 22–24 San Diego – CA.