The aim of this study was to conduct a cost-utility study of adefovir, entecavir, interferon alpha, pegylated interferon alpha, lamivudine and tenofovir for chronic hepatitis B in the context of Brazilian Public Health Care System. A systematic review was carried out for efficacy and safety. Another review was performed to collect utility data and transition probabilities between health states. A Markov model was developed in a time horizon of 40 years with annual cycles for three groups of: HBeAg positive, HBeAg negative, and all patients. These strategies were compared to a fourth group that received no treatment. Discount rates of 5% were applied and sensitivity analyses were performed. Tenofovir offered the best cost-utility ratio for the three evaluated models: U$397, U$385 and U$384 (per QALY, respectively, for HBeAg positive, negative, and all patients). All other strategies were completely dominated because they showed higher costs and lower effectiveness than tenofovir. The sequence of cost-utility in the three models was: tenofovir, entecavir, lamivudine, adefovir, telbivudine, pegylated interferon alpha, and interferon alpha. In the sensitivity analysis, adefovir showed lower cost-utility than telbivudine in some situations. The study has some limitations, primarily related to the creation of scenarios and modeling. In this study, tenofovir presented the best cost-utility ratio. The results obtained in this study will be valuable in decision-making and in the review of the clinical protocol, mainly involving the allocation of available resources for health care.
More than two billion people alive today have been infected with hepatitis B virus, and about 350 million remain infected. It is estimated that chronic hepatitis B is among the ten leading causes of death worldwide. Individuals with chronic hepatitis B have an increased risk of developing liver cirrhosis and hepatocellular carcinoma, causing the death of one million patients annually.1–3
Treatments of chronic hepatitis B aim to prevent or reduce the risk of liver cirrhosis and hepatocellular carcinoma. They also improve viral suppression, and seek to normalize ALT levels, decrease liver damage, and seroconversion of HBeAg (in HBeAg positive patients).1,3
Some of the pharmacological options for treating chronic hepatitis B include: interferon alpha (IFN-α), pegylated (PEG-IFN-α) and nucleoside/nucleotide analogs: lamivudine (LAM), entecavir (ETV), adefovir dipivoxil (ADF), telbivudine (LDT), and tenofovir disoproxil fumarate (TDF).
IFN-alpha is administered subcutaneously. It acts through two mechanisms in chronic hepatitis B: (i) antiviral action with direct inhibition of viral DNA synthesis; and (ii) immunomodulatory action. The nucleoside/nucleotide analogs are used orally and inhibit reverse transcription during the viral replication cycle in the hepatocyte.3
The incorporation of health technologies involves the introduction of new equipment, health products, and drugs. In general, new technology is expensive, bringing about the question of whether the improvement in results is significant vis-à-vis the aggregate cost of the new therapy. The introduction of economic evaluation methods applied to clinical decision making has been driven by the emergence of new drugs, the rise in pharmaceutical spending, the variability and uncertainty in clinical practice. Additionally, there is a need to prioritize the use of drugs aimed at improving quality of life of patients, contributed in the selection among pharmacological alternatives to be used in the health system. Economic evaluations in health care offer the possibility for a more rational use of drugs and better allocation of available resources; that is, they help determine how much more is needed to invest in a technology for an additional benefit.
The effectiveness and cost of treatments may differ in varying proportions. If the cost-effectiveness of each treatment is clearly analyzed, the proper allocation of resources becomes easier. The aim of this study was to conduct a cost-utility analysis of existing drugs for the treatment of chronic hepatitis B in the context of the Brazilian public health system.
Materials and methodsStudy designThe target population of this study was selected from a systematic review. It is composed of male and female adult patients with no history of resistance to antiviral drugs, and without coinfection with HIV, hepatitis C or hepatitis D virus. Patients were also tested for cirrhosis or hepatocellular carcinoma at the beginning of treatment.
The technologies evaluated in this study were the drugs for chronic hepatitis B in the usual doses used in Brazil, according to the clinical guideline:
- -
Adefovir dipivoxil: 10mg per day orally.
- -
Entecavir: 0.5mg per day orally.
- -
Interferon-α: 5MUI daily.
- -
Pegylated interferon-α: 1.5mcg/kg of pegylated interferon alpha 2b subcutaneously administered weekly (used as reference patients weighing 60kg).
- -
Lamivudine: 100mg per day orally.
- -
Telbivudine: 600mg per day orally.
- -
Tenofovir disoproxil fumarate: 300mg per day orally.
Patients receiving these drugs were compared to those receiving no treatment.
We considered patients with chronic hepatitis B who were followed up clinically, but did not receive treatment.
Clinical data searchA systematic review was performed for published articles that evaluate the efficacy and safety of treatments for chronic hepatitis B. It was conducted in five baselines: Cochrane Library, MedLine/PubMed, LILACS, SCIELO, and IPA (International Pharmaceutical Abstracts). We considered studies published in Portuguese, English, German or Spanish, published between 2000 and July 2011.
Two independent reviewers extracted data and assessed the trial's quality using the Jadad scale.4 Only studies of medium and high quality (at least 3 points on the Jadad scale) were included. Inclusion criteria were randomized controlled trials (RCTs) lasting for at least three months. We excluded review papers and editorials, case studies or studies with fewer than 10 patients.
Eligible studies were those conducted on adult patients older than 18 years, who had been diagnosed with chronic hepatitis B, received one of the drugs evaluated in the usual doses, and were compared with patients receiving other drugs, the association of two drugs, or a placebo. Studies, where the control group was given the same drug but in different doses or for a different duration of therapy, were excluded. In case of discrepancy in any of the enumerated steps, the discordant results were resolved by consensus between the reviewers and, when necessary, by a third reviewer.
From the selected articles, extracted data included: viral response, lack of response to treatment, treatment withdrawals, viral resistance, HBeAg seroconversion, viral breakthrough, and adverse events. Data were extracted on pre-prepared tables and separated into three groups: HBeAg positive patients, HBeAg negative patients, and a third group comprising all patients. This third group was created because some articles presented the aggregate results of HBeAg positive and negative patients.
Data about utility and transition probabilities between health statesAnother systematic review was conducted looking for studies that assessed the utility of patients with chronic hepatitis B in each condition. The transition probabilities of patients between health states were also extracted from these studies. This search was made in PubMed/MedLine. We also looked at studies that evaluated quality of life of patients at different stages of chronic hepatitis B, evaluated by a generic quality of life questionnaire.
ModelingThe TreeAge Pro® software was used to construct a decision-analytical Markov model with estimates of the natural history of chronic hepatitis B infection. Scenarios were created assuming health conditions and situations arising from the use of drugs and also from clinical pathology. The scenarios considered that the sequence of events that might occur would be the same for all interventions, carrying only the probability of events occurring in different treatments and also their costs and utilities.
The model was first applied to patients already diagnosed with chronic hepatitis B infection. The decision node represents the patient's treatment options (ADF, ETV, IFN-α, LAM, TDF, LdT and PEG-IFN-α) or no treatment. The treatment was considered discontinued in the states, “serious adverse events” or “death”. Furthermore, it was considered that in the states, “no response to treatment” and “viral resistance,” the treatment was changed to TDF, as recommended by the Brazilian Guideline (for patients who received TDF, this was changed to ETV).
For the state, “death,” mortality data from the Brazilian general population was used, considering patients aged 40 years in whom mean disease onset was found in the systematic review.
After building the model, the yearly costs of each stage were added. The data were extrapolated to a time horizon of 40 years, considering the chronic nature of the disease. We performed a cost-utility analysis, which measures the cost of treatment related to QALY.5 The incremental cost-effectiveness ratio (ICER) was evaluated to compare the alternatives that were more expensive and more effective than others.
CostsTo evaluate the cost of each treatment, the parameters of the Brazilian public health system were used; only direct costs were included. The cost of the drugs were obtained by averaging the amounts paid by the Ministry of Health in the bids made during the year 2011, with the exception of the LdT, which is not funded by the Brazilian public health system. For this product, the ABC Pharma table was used, which standardizes the selling price of medicines in the private network in the country. For the cost of other procedures (consultations, examinations, hospitalizations and surgeries), we used the cost table produced by the Brazilian health system hospitals.
Discount rateThe discount rate used in the study was 5%, both for cost and effectiveness values.
Sensitivity analysisThe uncertainties were minimized with sensitivity analyses that were performed using univariate analysis, varying the parameters of cost and effectiveness for more and less. To evaluate the robustness of the method and reduce uncertainties, the following types of analyses were performed:
- -
Change the weight of the patients using PEG-IFN-α 2b from 60 to 70kg; ranged the utilities of treatment as reported in the literature.
- -
Variation of the cost of each treatment according to the maximum and minimum prices paid for each drug in the tenders held during 2011.
- -
Discount rates varied between 0 and 10%.
- -
Transition probabilities between health states were varied from minimum and maximum values found in literature.
In the first stage of the systematic review, 1174 articles published between 2000 and July 2011 were found in the databases. Of these, 100 randomized clinical trials were selected based on their abstracts. Of these, 246–29 studies met the inclusion criteria and were included.
We included data only from studies that evaluated the patients during one year. The extracted probabilities were described in Table 1 for HBeAg positive, negative, and all patients. For HBeAg negative patients, we found no RCTs that evaluated the IFN-α alone, without association, and met the inclusion criteria. Therefore, for this group of patients, the drug was not evaluated.
Probabilities of patients reaching each health state, starting from the state “chronic hepatitis B” for each of the treatments.
| Drug | Viral responsea | HBeAg seroconversion | Viral breakthrough | Viral resistance | Withdrawals due to adverse events | No response |
|---|---|---|---|---|---|---|
| HBeAg positive patients | ||||||
| ADF9,10,26 | 0.2175 | 0.1127 | 0.0909 | 0.0186 | 0.0150 | NR |
| ETV7–9 | 0.6589 | 0.2041 | 0.0169 | NR | 0.0026 | 0.0537 |
| IFN-α11,13 | 0.1756 | 0.1646 | 0.0625 | NR | 0.0405 | NR |
| PEG-IFN-α12,16 | 0.1820 | 0.2433 | NR | NR | 0.0364 | NR |
| LAM11,16 | 0.3741 | 0.1979 | 0.1655 | 0.1188 | 0.0302 | 0.1186 |
| LdT18,19,26 | 0.6154 | 0.2262 | 0.0631 | 0.0569 | 0.0000 | 0.0463 |
| TDF10 | 0.8312 | 0.2092 | 0.0235 | NR | 0.0000 | NR |
| HBeAg negative patients | ||||||
| ADF10,17,20,26 | 0.5917 | – | 0.0909 | 0.0186 | 0.0081 | NR |
| ETV22 | 0.9015 | – | 0.0169 | NR | 0.0185 | 0.0537 |
| IFN-α | NR | – | NR | NR | NR | NR |
| PEG-IFN-α10,21,24 | 0.6407 | – | NR | NR | 0.0606 | NR |
| LAM10,18,23,24 | 0.7312 | – | 0.1224 | 0.1061 | 0.0465 | 0.0268 |
| LdT18,19,26 | 0.8802 | – | 0.0225 | 0.0225 | 0.000 | 0.0045 |
| TDF10 | 0.9320 | – | 0.0235 | NR | 0.0200 | NR |
| All patients | ||||||
| ADF9,10,17,20,25,26 | 0.3748 | 0.1127 | 0.0909 | 0.0186 | 0.0132 | NR |
| ETV7–9,22 | 0.7697 | 0.1909 | 0.0169 | NR | 0.0132 | 0.0537 |
| IFN-α11,13 | 0.1756 | 0.1646 | 0.0625 | NR | 0.0405 | NR |
| PEG-IFN-α10,12,16,21,24 | 0.3227 | 0.2433 | NR | NR | 0.0438 | NR |
| LAM6,10–16,18,23,24 | 0.5140 | 0.1979 | 0.1567 | 0.1152 | 0.0241 | 0.1013 |
| LdT18,19,26 | 0.6872 | 0.2262 | 0.0528 | 0.0482 | 0.0000 | 0.0351 |
| TDF10 | 0.8592 | 0.2092 | 0.0235 | NR | 0.0117 | NR |
ADF, adefovir dipivoxil; ETV, entecavir; IFN-α, interferon alpha; PEG-IFN-α, pegylated interferon alpha; LAM, lamivudine; LdT, telbivudine; TDF, tenofovir disoproxil fumarate; NR, not related.
31 articles were found in the systematic review performed to search for the utilities of patients with chronic hepatitis B in different health states. Of these, only three articles met the inclusion criteria and were original, while in the other 28 papers the utilities were taken from another study. Of the three resulting articles, the study by Levy et al.30 was chosen for the extraction of utility values, because it was a multi-center study conducted on 1134 patients, on whom the Health Related Quality of Life Questionnaire (HRQOL) was conducted. The utility values found for each state of health were (range):
- -
Uncomplicated chronic hepatitis B: 0.68 (from 0.66 to 0.70).
- -
Compensated cirrhosis: 0.69 (0.66–0.71).
- -
Decompensated cirrhosis: 0.35 (0.32–0.37).
- -
Hepatocellular carcinoma: 0.38 (0.36–0.41).
- -
Liver transplantation (first year): 0.57 (0.54–0.60).
- -
Liver transplantation (other years): 0.67 (0.64–0.69).
Some utility values were not found in the literature and therefore were adapted from the selected study. For the outcomes “viral response” and “HBeAg seroconversion”, values were considered equivalent to the value “health”. The outcomes “no response to treatment,” “withdrawal due to adverse events,” and “viral resistance” were considered equivalent to the value “uncomplicated chronic hepatitis B”.
Transition probabilitiesThe transition probabilities between the health states of chronic hepatitis B were also extracted from the studies selected in the systematic review,2,31–37 obtaining the average, maximum and minimum values. The values are described in Table 2.
Probabilities of transition between the health status of chronic hepatitis B extracted from the studies of the systematic review.
| Initial state | Final state | Probability (range) | References |
|---|---|---|---|
| Chronic hepatitis B | Compensated cirrhosis | 0.0488 (0.02–0.09) | 28,30–33 |
| Hepatocellular carcinoma | 0.0115 (0.008–0.015) | 9,28–33 | |
| Death | 0.0014 | 2 | |
| HBeAg seroconversion | 0.0160 | 32 | |
| Viral resistance | Death | 0.0290 | 28 |
| Seroconversion | Compensated cirrhosis | 0.0391 | 28 |
| Hepatocellular carcinoma | 0.0030 | 9,30 | |
| Compensated cirrhosis | Decompensated cirrhosis | 0.0572 (0.031–0.099) | 28–33 |
| Hepatocellular carcinoma | 0.0391 (0.02–0.071) | 29–33 | |
| Death | 0.0500 (0.049–0.051) | 28,29,32 | |
| Hepatocellular carcinoma | Liver transplantation | 0.0008 | 32 |
| Death | 0.3173 (0.233–0.433) | 29,33 | |
| Decompensated cirrhosis | Hepatocellular carcinoma | 0.0652 (0.022–0.034) | 30–33 |
| Liver transplantation | 0.0260 (0.014–0.05) | 30–33 | |
| Death | 0.2506 (0.144–0.39) | 29–33 | |
| Liver transplantation | Death | 0.1098 (0.069–0.15) first year | 30,31 |
| 0.0217 (0.015–0.025) following years | 30,31 | ||
In untreated patients the following probabilities were considered:33,38
- •
Compensated cirrhosis: 23.89%.
- •
Clinical remission: 1.6%.
- •
Death: 2.9%.
- •
Hepatocellular carcinoma: 1.5%.
- •
Hepatocellular carcinoma to death: 50%.
Costs of drugs per patient over a one year treatment period are shown in Table 3. We calculated the average cost of each drug in the year 2011, considering that the price can vary between the bids. The cost of IFN-α and PEG-IFN-α was added in the Markov model only for the first year because Brazilian guidelines prohibit therapy with interferon beyond one year.
Drugs annual cost.
| Drug | Annual doses | Average cost per dose (U$) | Minimum cost per dose (U$) | Maximum cost per dose (U$) | Average annual cost (U$) | Annual minimum cost (U$) | Annual maximum cost (U$) |
|---|---|---|---|---|---|---|---|
| ADF 10mg | 365 | 3.41 | 3.13 | 3.92 | 1246 | 1142 | 1432 |
| ETV 0.5mg | 365 | 5.06 | 4.91 | 5.30 | 1266 | 1791 | 1933 |
| IFN-α 2B 5000UI | 156 | 20.55 | 20.55 | 20.55 | 3206 | 3206 | 3206 |
| LAM | 365 | 0.32 | 0.32 | 0.32 | 117 | 117 | 117 |
| PEG-IFN-α 2b 100mcg | 52a | 220.93 | 220.93 | 220.94 | 11,488 | 11,488 | 11,489 |
| LdT | 365 | 13.63 | 11.31b | 15.95b | 4976 | 4130 | 5823 |
| TDF | 365 | 2.09 | 1.74 | 2.34 | 763 | 634 | 853 |
For the cost of health states defined by decompensated cirrhosis, hepatocellular carcinoma and liver transplantation, values were taken from another study conducted in Brazil in 2005, for which the price list has not been updated since then:39
- -
Decompensated cirrhosis: U$ 12,803.
- -
Hepatocellular carcinoma: U$ 2770. This cost does not take into consideration chemotherapy.
- -
Liver transplantation: U$ 50,798 in the first year and subsequent years we used the cost of clinical care.
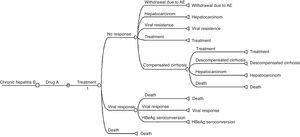
Three Markov models were developed: for HBeAg positive, negative, and for all patients (Fig. 1). The main difference in the model of HBeAg negative patients is that there is no seroconversion of HBeAg.
It was considered that all patients started in the state “treatment” and then transited to one of the health states: viral response, death, no response to treatment, withdrawal due to adverse events, seroconversion of HBeAg (in HBeAg positive patients), viral resistance, compensated cirrhosis, or hepatocellular carcinoma.
Cost-utility analysesThe results of cost-utility analyses are shown in Fig. 2, for HBeAg positive, negative and all patients, respectively. The costs, effects, and cost-utility are described in Table 4. The results are in increasing order of cost-utility values, and compared to the item “no treatment”.
Cost-utility results in ascending order of cost-utility ratio.
| Drug | Cost (U$) | Incremental cost (U$) | Effect (QALY) | Incremental effect (QALY) | Cost-utility (U$/QALY) | Incremental cost-utility |
|---|---|---|---|---|---|---|
| HBeAg positive patients | ||||||
| TDF | 4721 | −14,293 | 11.87 | 4.13 | 397 | |
| ETV | 6399 | −12,615 | 11.80 | 4.06 | 542 | Dominated |
| LAM | 6723 | −12,291 | 10.37 | 2.63 | 648 | Dominated |
| ADF | 11,775 | −7239 | 11.06 | 3.32 | 1064 | Dominated |
| LdT | 15,796 | −3218 | 11.50 | 3.76 | 1371 | Dominated |
| No treatment | 19,014 | – | 7.74 | – | 2456 | Dominated |
| PEG-IFN-α | 41,906 | 22,892 | 11.34 | 3.60 | 3695 | Dominated |
| IFN-α | 42,534 | 23,520 | 11.10 | 3.36 | 3832 | Dominated |
| HBeAg negative patients | ||||||
| TDF | 4592 | −14,471 | 11.92 | 4.18 | 385 | |
| ETV | 5509 | −13,554 | 11.91 | 4.17 | 462 | Dominated |
| LAM | 5653 | −13,410 | 10.92 | 3.19 | 518 | Dominated |
| ADF | 7894 | −11,169 | 11.50 | 3.77 | 686 | Dominated |
| LdT | 8933 | −10,130 | 11.88 | 4.14 | 752 | Dominated |
| PEG-IFN-α | 15,218 | −3845 | 11.81 | 4.07 | 1289 | Dominated |
| No treatment | 19,063 | – | 7.74 | – | 2463 | Dominated |
| All patients | ||||||
| TDF | 4664 | −14,399 | 11.85 | 4.11 | 394 | |
| ETV | 5896 | −13,167 | 11.83 | 4.09 | 498 | Dominated |
| LAM | 5919 | −13,144 | 10.75 | 3.01 | 551 | Dominated |
| ADF | 9511 | −9552 | 11.16 | 3.43 | 852 | Dominated |
| LdT | 13,639 | −5424 | 11.58 | 3.84 | 1178 | Dominated |
| No treatment | 19,063 | – | 7.74 | – | 2463 | Dominated |
| PEG-IFN-α | 28,817 | 9754 | 11.54 | 3.80 | 2497 | Dominated |
| IFN-α | 42,141 | 23,078 | 11.01 | 3.27 | 3827 | Dominated |
PEG-IFN-α, pegylated interferon-alpha; IFN-α, interferon-alpha; QALY, quality adjusted life years.
By varying the initial utility or utility of the state viral response in HBeAg negative patients, in some cases, PEG-IFN becomes less cost-effective than the option “no treatment”. Varying the cost of LdT in HBeAg negative patients, LdT shows better cost-utility values than ADF. In the three groups of patients, varying the discount rate to 10%, LAM was more cost-utility than ETV.
Because TDF had the best cost-utility of the evaluated drugs, the worst scenario was created for TDF based on data from systematic reviews. The probability of developing hepatocellular carcinoma, compensated cirrhosis, and decompensated cirrhosis was ranged to the highest value found in the literature. Still, TDF was the alternative with the better cost-effectiveness ratio among the three groups of evaluated patients; all other strategies were dominated.
DiscussionThe cost-utility of TDF for the treatment of chronic hepatitis B was higher than for the other drugs in all groups of patients (HBeAg positive, negative and total). All other strategies were dominated, that is, all other treatment strategies had higher cost and lower effect than TDF. ETV was the second most cost-effective drug in all groups. In the three groups studied, the TDF and ETV QALY values were very close. However, due to the lower cost of TDF, its cost-effectiveness ratio was better when compared to ETV.
IFN-α was the strategy with the worst cost-effectiveness ratio in HBeAg positive patients and the group comprising all patients, and also was less cost-effective than the no treatment option. Moreover, its use in HBeAg positive patients should be evaluated, especially in the presence of cirrhosis. In this case, treatment with IFN-α can lead to serious complications such as neutropenia, sepsis and hemorrhages.40 Further, this treatment leads to a high proportion of adverse events (about 4%) and showed lowest rates of viral response (Table 1). Patients without viral load reduction and/or HBeAg seroconversion are more likely to develop liver cirrhosis and hepatocellular carcinoma, which is associated with higher costs in treatment.41
Among the other treatment options, PEG-IFN had the worst cost-utility ratio in HBeAg negative patients. In the other two groups, PEG-IFN was only better than IFN. However, PEG-IFN-α appears to have some important advantages when compared to nucleosides/nucleotides analogs, because it does not lead to viral resistance, has post-treatment effect, and its duration therapy is generally finite. However, it also has some disadvantages, such as requiring injection, less potent suppression of HBV DNA, and an increase in adverse events.40
LAM had the worst QALY values in HBeAg positive and all patients groups. However, in both groups, LAM was the third most cost-effective product. This is due to lower cost of LAM compared to other drugs. Of all evaluated drugs, LAM had the lowest cost at U$ 117.
The study was considered robust by the constancy in their univariate results despite changes in some parameters considered important for the analysis (cost and effectiveness), because in all cases, TDF was more cost-effective and dominated the other treatment options.
In recent years, health spending has risen and become a problem in developed countries and particularly in developing countries. The causes of increases in spending are diverse and may be related to the emergence of new technologies, often more effective but more expensive. Moreover, with the emergence of new diagnostic and therapeutic strategies, life expectancy is rising, increasing the number of elderly and therefore overall spending on health. There is a continuous struggle by health systems to maximize health gains with the use of available resources. Economic evaluations are critical in determining the best allocation of resources, looking for the most cost-effective alternatives especially in the treatment of chronic diseases.
Such use of economic evaluations has increased significantly worldwide. Several economic evaluations have been performed in order to compare the cost-utility of treatments for chronic hepatitis B, aiming at a better allocation of resources in health.
In Kanwall's study conducted in the United States in 2005, the treatment strategies with ADF, LAM and IFN were compared with no treatment. IFN was the most cost-effective drug among the three, both in HBeAg positive and negative patients, and the ADF and LAM were dominated strategies.33 Another study performed in Asia in 2008, compared LAM, ETV, and LAM in association with ADF, in HBeAg positive patients. ETV was more cost-effective in reducing levels of HBV DNA and HBeAg seroconversion.42 In Brazil, the ETV was compared directly to LAM in a study in 2008. The study was conducted in the context of the Brazilian public health system on a time horizon of ten years. For HBeAg positive and HBeAg negative patients, the ETV was more cost-effective when compared to LAM.43 In the United States, in 2008, Veenstra et al. evaluated the cost-effectiveness of treatments with PEG-IFN or LAM for chronic hepatitis B in HBeAg negative patients. PEG-IFN-α was more cost-effective than LAM.36 A Spanish study conducted in 2009 assessed the cost-effectiveness ratio between the drugs ADF, ETV, LAM, LdT, and TDF in HBeAg positive and HBeAg negative patients. TDF was the most cost-effective drug in both groups (HBeAg positive and negative).44 In 2010, an economic evaluation carried out in Lithuania, compared PEG-IFN-α with IFN-α and with LAM, for the treatment of chronic hepatitis B in HBeAg positive and negative patients. PEG-IFN-α was the most cost-effective drug among the three.45
Despite several economic evaluations already carried out worldwide, a comparison of costs among different countries is difficult to achieve, since every nation has its own unique tax burden and economic structure. Data from studies conducted in other countries generally cannot be extrapolated.
The incorporation of new technologies must be done appropriately in health care in order to generate the best impact on the health of the population. It is important to note that cost-utility assessments provided by different health technologies serve to guide the manager and the clinician in his choice of alternatives. Health decisions cannot be treated as part of the exact sciences, and they must be rational and supported in the data, but cannot be as extreme as to eliminate alternatives based solely on numerical data.
LimitationsThe modeling for economic evaluations can bring limitations because values are extrapolated based on findings in the literature and created scenarios. Sensitivity analyses are performed to try to minimize possible variations in the model. However, some limitations should be considered.
First, the results of clinical outcomes of this study were derived from clinical studies from the last ten years. Clinical trials evaluate the efficacy of therapies, and not effectiveness, which reflect the clinical practice in use. The course of chronic HBV infection is dynamic, which could be modified significantly with anti-HBV agents but it is still dynamic. We used data extracted from systematic review that only had limited therapy interval and limited follow-up duration to estimate the long-term cost-effectiveness of drug therapy. Moreover, there might be significant differences in the HBeAg seroconversion rate and viral resistant rate in the 1st and 2nd year of treatment. Thus, one year data could lead to significant bias. However, these data were used because there are no clinical studies available for all these drugs with more than one year duration.
The population involved in clinical trials is heterogeneous, and the responses to treatments may be different in our population. Also, different genotypes were found in the included studies, which are not necessarily the same genotypes found in the Brazilian population.
Another important limitation of the study is that in the Markov model, it was assumed that the patient cannot be in two health states at the same time. This assumption differs from reality because patients could, for example, have decompensated cirrhosis and hepatocellular carcinoma at the same time, and still experience a serious adverse event. However, the model precludes the representation of this type of a situation because the clinical trials report the data separately.
TDF was found to be the drug with the best cost-utility ratio for the three groups of patients. However, this information is ascertained only from two studies, as described in the article by Marcellin et al.11 A larger number of studies may provide a more reliable result. New clinical studies of TDF would, therefore, increase the robustness of this result.
The results of this study establish the cost-utility of drugs available in Brazil used to treat chronic hepatitis B. Understanding this relationship is crucial for health professionals; it bolsters the decision-making process. It is essential for doctors to be aware of the cost-utility ratios of each drug. However, each patient responds to therapy in different ways and should be evaluated individually.
Conflict of interestThe authors declare no conflict of interest.