Acinetobacter spp. have emerged as notorious pathogens involved in healthcare-associated infections. Carbapenems are important antimicrobial agents for treating infections due to multidrug resistant Acinetobacter spp. Different mechanisms may confer resistance to these drugs in the genus, particularly production of class D carbapenemases. OXA-23-like family has been pointed out as one of the predominant carbapenamases among Acinetobacter. The present work aimed to investigate the occurrence of OXA-23-like carbapenemases among Acinetobacter isolates recovered from patients of a university hospital in Niterói, RJ, Brazil.
MethodsAntimicrobial susceptibility profiles were determined by disk-diffusion. Imipenem resistant isolates were submitted to Modified Hodge Test in order to screen for carbapenemase production, and later to polymerase chain reaction (PCR) to investigate the presence of blaOXA-23.
ResultsImipenem and meropenem resistance rates were 71.4% and 69.7%, respectively. The Modified Hodge Test revealed carbapenemase production among 76 (89.4%) of the 85 imipenem resistant isolates analyzed; according to PCR results, 81 isolates (95.4%) carried the blaOXA-23 gene.
ConclusionsOXA-23-like enzymes may be an important mechanism of carbapenem resistance among isolates present in the hospital studied.
Acinetobacter spp. have emerged as one of the most important pathogens involved in health care associated infections in recent decades. These non-fermentative Gram-negative cocobacilli are frequently involved in the etiology of ventilator-associated pneumonia, bacteremia, urinary tract infections, and surgical site infections. These pathogens are also notorious for their ability to accumulate different mechanisms of antimicrobial resistance, often showing a multidrug-resistant phenotype.1–4
Carbapenems are considered important antimicrobial agents for treating infections due to multidrug-resistant Acinetobacter spp. However, reports of resistance to these drugs have emerged, with increasing frequency, among Acinetobacter spp. clinical isolates.5–10
Different mechanisms may confer carbapenem resistance in Acinetobacter spp., but production of carbapenemases is considered the most important one, particularly those belonging to Ambler's class D, also known as oxacilinases (OXA). The class B carbapenemases or metallo-β-lactamases (MβLs) can also be found among Acinetobacter spp., although less frequently.11
Five major groups of OXA with carbapenemase activity have been identified in Acinetobacter baumannii: acquired OXA-23-like, OXA-40-like, OXA-58-like, and OXA-143-like families, and the OXA-51 group, which codifies a chromosomal oxacilinase intrinsic to A. baumannii. When overexpressed, these enzymes can confer carbapenem resistance.12–17
Among OXA, the variants comprising the OXA-23-like family have been detected throughout the world, and have also been pointed out as the predominant carbapenamases among Acinetobacter in several geographic regions.
In Brazil, the first report of isolates producing these enzymes were in the city of Curitiba, Paraná.15,18–23 After that report, Acinetobacter spp. clinical isolates producing OXA-23-like enzymes have been also identified in cities like Porto Alegre-RS, Rio de Janeiro-RJ, Niterói-RJ, São Paulo-SP, Belo Horizonte-MG, Blumenau-SC, and São Luís-MA.24–31 The OXA-143-like enzymes, as well as OXA-72 and OXA-58, have also been found in Brazil.12,16,17,27,32
The present study aimed to investigate the occurrence of isolates producing the OXA-23-like carbapenemases among imipenem and/or meropenem-resistant Acinetobacter isolates, isolated at a university hospital in Niterói, RJ, Brazil.
Material and methodsBacterial strainsA total of 119 Acinetobacter spp. clinical isolates were studied. They were recovered between July 2007 and July 2009, from different clinical specimens of 100 patients admitted to Hospital Universitário Antônio Pedro (HUAP), a 290-bed public tertiary teaching hospital. Isolates recovered from the same patient that were isolated from different infection sites, at different periods of time, or that showed distinct antimicrobial susceptibility profiles were included in the study.
The most frequent source of isolation was the inferior respiratory tract (n = 44; 37%), followed by blood (n = 28; 23.5%), urine (n = 17; 14.3%), and catheter tip (n = 14; 11.8%). Skin and ascitic fluid isolates corresponded to 5% (n = 6) and 2.5% (n = 3) of the entire collection, respectively. Other specimens represented 4.2% (n = 5), whereas two isolates could not have their origin determined (1.7%). The intensive care unit (ICU) was the hospital ward that showed the greatest number of isolates (68/119; 57.1%).
Bacterial strains identificationThe identification of the isolates at the genus level was performed using the VITEK 1 (bioMérieux - Marcy l’Etoile, France) automated system, at the Microbiology Laboratory of the Pathology Service of HUAP.
Antimicrobial susceptibility profilesThe antimicrobial susceptibility profiles of clinical isolates were determined by disk-diffusion, according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI – 2009).33 The following antimicrobials (CECON – São Paulo, Brazil) were tested: amikacin (30μg), ampicillin/sulbactam (10μg), cefepime (30μg), ceftazidime (30μg), ciprofloxacin (5μg), gentamicin (10μg), imipenem (10μg), meropenem (10μg), piperacillin/tazobactam, (75mg/10μg), sulfamethoxazole/trimethoprim (23.75/1.25μg), ticarcillin/clavulanate (75/10μg), and tetracycline (30μg). Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used as quality control strains.
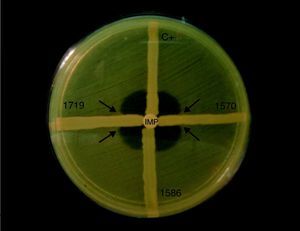
Phenotypic detection of carbapenemases productionImipenem resistant (IMP-R) isolates were submitted to Modified Hodge Test, as described previously by Lee et al. (2001), with the modifications indicated bellow.34
The surface of a Mueller-Hinton agar plate was inoculated with a bacterial suspension made of E. coli ATCC 25992 fresh cultures (0.5 McFarland turbidity). A 10μg imipenem disk was placed at the center of the plate. Then, streaks of the imipenem-resistant isolates were performed from the edge of the disk to the edge of the plate. The test result was considered positive if the presence of a distorted inhibition zone was observed after incubation at 37°C during 16h to 18h. Acinetobacter spp. strain A29009 producing OXA-23 was used as positive control.
Detection of blaOXA-23All imipenem resistant isolates were investigated by PCR in order to determine the presence of blaOXA-23, according to Woodford et al. with the modifications described below.35
DNA extraction was accomplished by boiling a 3.0 Mc-Farland turbidity suspension of each isolate for 10minutes. Then, these suspensions were centrifuged at 8,000rpm for 2minutes and the supernatant was used as template in PCR experiments. A 2 μL volume of DNA template was added to 23 μL of reaction mixture, which contained 2.5 μL of PCR buffer 10x (10mM Tris HCl, 25mM KCl), 1mM of MgCl2, 0.5μM of each primer, 100μM of each deoxynucleotide trifosfate (dATP, dGTP, dCTP, dTTP) and 1.5U of Taq polimerase (Invitrogen – São Paulo, Brazil). The sequences of primers used were: GATCGGATTGGAGAACCAGA and ATTTCTGACCGCATTTCCAT.30The reaction was carried as follows: initial denaturation at 94°C for 5minutes; followed by 30 cycles of 94°C for 1minute, 54°C for 1minute, 72°C for 1 minute; and final extension of 72°C for 5minutes. A PCR product of 501pb was expected. Acinetobacter spp. strain A 29009 producing OXA-23 was used as positive control for the amplification experiments.
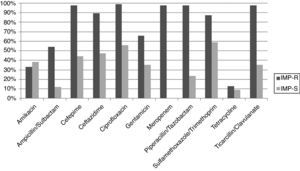
ResultsAntimicrobial susceptibility profilesHigh rates of resistance were observed among isolates studied. Resistance rates ≥ 70% were observed for seven of the 12 antimicrobial agents tested (Table 1). The highest resistance rate was observed for ciprofloxacin (86.6%), followed by cefepime (82.3%). The percentages for carbapenems were also elevated; 71.4% for imipenem and 69.7%, for meropenem.
Antimicrobial susceptibility profile of 119 Acinetobacter spp. clinical isolates recovered between July 2007 and July 2009, at the Hospital Universitário Antônio Pedro, in Niterói, RJ, Brazil.
| Antimicrobial | Resistantn (%) | Intermediaten (%) | Sensitiven (%) |
|---|---|---|---|
| Amikacin | 41 (34.5) | 8 (6.7) | 70 (58.8) |
| Ampicillin/sulbactam | 50 (42) | 21 (17.7) | 48 (40.3) |
| Cefepime | 98 (82.3) | 2 (1.7) | 19 (16) |
| Ceftazidime | 92 (77.3) | 2 (1.7) | 25 (21) |
| Ciprofloxacin | 103 (86.6) | - | 16 (13.4) |
| Gentamicin | 68 (57.1) | - | 51 (42.9) |
| Imipenem | 85 (71.4) | - | 34 (28.6) |
| Meropenem | 83 (69.7) | - | 36 (30.3) |
| Piperacillin/tazobactam | 91 (76.5) | 2 (1.7) | 26 (21.8) |
| Sulfamethoxazole/trimethoprim | 94 (79) | 4 (3.4) | 21 (17.6) |
| Ticarcillin/clavulanate | 95 (79.8) | 2 (1.7) | 22 (18.5) |
| Tetracycline | 14 (11.8) | 39 (32.8) | 66 (55.4) |
The isolates showed the highest susceptibility rates to amikacin, followed by tetracycline and gentamicin (58.8%, 55.4%, and 42.9%, respectively). Of notice, a relatively high rate of intermediate resistance was observed for tetracycline (32.8%) and ampicillin/sulbactam (17.7%).
IMP-R strains were isolated throughout the entire study period and showed more elevated resistance rates to other antimicrobials than isolates susceptible to this antimicrobial (Fig. 1). Among IMP-R isolates, the highest susceptibility rate was also observed for amikacin (60%).
Most carbapenem resistant isolates were recovered from patients hospitalized at the ICU (80%; 68/85), and were recovered mostly from the inferior respiratory tract, (n = 35; 41.2%), followed by blood (n = 21; 25.7%), urine (n = 11; 12.9%), and catheter tip (n = 9; 10.6%).
Detection of carbapenemase production and blaOXA-23The Modified Hodge Test results revealed carbapenemase production among 76 (89.4%) of the 85 IMP-R Acinetobacter isolates analyzed (Fig. 2).
According to results obtained by PCR, 81 (95.4%) of the 85 IMP-R isolates carried the blaOXA-23 gene (Fig. 3).
Detection of blaOXA-23-like genes in Acinetobacter spp. clinical isolates. Lines 1 and 21: 1 Kb Plus DNA Ladder; line 2: blaOXA-23 positive control; line 3: blaOXA-23 negative control; lines 4-14 and 16-19: isolates carrying blaOXA-23; line 15: isolate not carrying blaOXA-23; line 20: reaction control.
Six isolates carrying blaOXA-23 showed negative results for modified Hodge Test. Conversely, one isolate was positive for this phenotypic test, but the presence of blaOXA-23 was not detected. One of the two isolates that were susceptible to meropenem and resistant to imipenem carried the blaOXA-23 gene.
DiscussionAll isolates evaluated in this study have been identified as belonging to the A. baumanni-complex by the automated system. However, it is well established that this method is not suitable to distinguish between A. baumannii and other species such as Acinetobacter pitii, Acinetobacter nosocomialis, and Acinetobacter calcoaceticus.36 The presence of blaOXA-51 in all isolates was also investigated, with positive results (data not shown). Although blaOXA-51 detection has been pointed out as a simple and reliable way of identifying A. baumannii, this gene has also been found in A. nosocomialis, jeopardizing this presumptive bacterial identification at the species level.37,38 For the abovementioned reasons, bacterial identification results are expressed only at the genus level in this study.
High resistance rates were observed for most of the antimicrobials agents studied, including carbapenems, and even more elevated rates were observed among IMP-R isolates. Antimicrobial resistance considerably restricts the available treatment options, especially resistance to carbapenem, which is considered to be the first option to treat severe infections due to Acinetobacter spp.
Modified Hodge Test results showed good correlation with PCR (96.2%), since only six blaOXA-23-positive isolates could not be detected using the phenotypic methodology, suggesting that this phenotypic technique is appropriate for screening Acinetobacter spp. isolates producing such carbapenemases. These results were consistent with those of Yang et al, which observed 100% positivity in Modified Hodge Test among A. baumannii strains that were IMP-R and OXA-23 producers.20 Recently, researchers from Rio de Janeiro pointed out that 87.6% of IMP-R isolates carrying blaOXA-23 were detected as carbapenemase producers by the Modified Hodge Test.24
In the present study, one isolate showing positive Modified Hodge Test result did not carry the blaOXA-23 gene, which could suggest either a false-positive phenotypic result or the presence of another carbapenemase type. Metallo-β-lactamases may be involved in carbapenem resistance phenotype among Acinetobacter spp., although less frequently than class D enzymes. In addition, coproduction of metallo-beta-lactamases and oxacilinases has been described in Acinetobacter spp. In this study, phenotypic screening tests were conducted to assess the production of metallo-β-lactamases,39 although no isolate showed positive results (data not shown). Thus, the mechanism involved in carbapenem resistance in the abovementioned isolate remains to be elucidated.40,41
A high frequency of blaOXA-23 (95.4%) was observed among IMP-R isolates analyzed. This result implies that production of OXA-23-like enzymes may be an important mechanism of carbapenem resistance among isolates present in the hospital studied.
The detection of A. baumannii producing OXA-23 is frequent in many countries, suggesting a worldwide dissemination of this enzyme.21 According to Kempf and Rolain, Europe, Singapore, Australia, the USA, Algeria, Egypt, Libya, South Africa, Thailand, Tunisia, Iraq, and French Polynesia reported either nosocomial outbreaks or sporadic cases of Acinetobacter producing OXA-23.42 In Asia, the most prevalent disseminated class D enzyme amongst A. baumannii in China and Korea is OXA-23.43 In Latin America, OXA-23 was identified in A. baumannii isolates from Argentina, Colombia, and Brazil.30 In Brazil, OXA-23-like enzymes have been identified as one of the major carbapenem resistance mechanism in Acinetobacter spp.25,28–32,44,45 In Rio de Janeiro, a surveillance study conducted in eight hospitals showed a wide dissemination of isolates producing such carbapenemases. In Niterói, the occurrence of A. baumannii strains carrying blaOXA-23 has already been reported at a public hospital, however, at a smaller rate (15%) than found in the present study.24,27
In conclusion, early detection of IMP-R Acinetobacter isolates, and of the corresponding mechanism of resistance is extremely important to prevent their dissemination throughout the hospital environment. It could be beneficial to routinely perform the surveillance of isolates producing carbapenemases in the hospital investigated. In addition, strict control measures, as well as prudent utilization of antimicrobials agents should be continuously promoted.
Conflict of interestAll authors declare to have no conflict of interest.
To Dr. Ana C. Gales (Universidade Federal de São Paulo, São Paulo, Brazil) for providing OXA-23 positive control strain.