The aim of this study was to explore the role of cytokines in the pathogenesis of hemorrhagic fever with renal syndrome (HFRS).
MethodsDouble-antibody sandwich ELISA was used to determine serum interleukin (IL)-6, urine tumor necrosis factor (TNF), IL-6, and IL-8 levels in 56 patients with HFRS.
ResultsSerum IL-6, urine TNF, IL-6, and IL-8 concentrations in HFRS patients were significantly higher than those in the control group (p<0.001). The concentrations increased at fever stage, then continued to increase during the hypotension stage and peaked at the oliguria stage. The concentrations of serum IL-6, urine TNF, IL-6, and IL-8 increased according to the severity of the disease, and differed greatly among different types of the disease. Serum IL-6 had remarkable relationships with serum specific antibodies. It was positively related to serum β2-microglobulin (β2-MG), blood ureanitrogen (BUN), and creatinine (Cr). Significant positive relationships were also found both between urine IL-6 and TNF, and between IL-6 and IL-8 (r=0.5768, p<0.05; r=0.3760, p<0.01).
ConclusionTNF, IL-6, and IL-8 were activated during the course of the disease. IL-6 was associated with the immunopathological lesions caused by the hyperfunction of the humoral immune response. IL-6, IL-8 and TNF were involved in renal immune impairment. Determining them might, to a certain extent, be useful in predicting the prognosis and outcome of patients with HFRS.
Recent studies have shown that immunomodulation abnormities have a significant role in hemorrhagic fever with renal syndrome (HFRS). The hyperfunction of humoral immune response causes excessive generation of antigen-antibody complexes, leading to secondary immune reaction. It also causes hypofunction of stimuli, increase in CD8T+ cells, and cellular immunomodulation dysfunction.1–3 The dynamic change of the concentrations of serum interleukin-6 (IL-6), urine tumor necrosis factor (TNF), IL-6, and IL-8 in patients with HFRS were detected by ELISA. The relationships of IL-6 with serum specific IgM and IgG antibodies, and with β2-microglobulin (β2-MG), respectively, were also analyzed to explore the role of IL-6 in the pathogenesis of HFRS.
Methods and patientsSubjectsThe subjects were 56 inpatients with positive HFRS-specific IgM and IgG antibodies, admitted to the Department of Infectious Diseases of this hospital and of the Xi’an Infectious Disease Hospital from 1992 to 2003. This study was conducted in accordance with the Declaration of Helsinki, with approval from the Ethics Committee of the First Affiliated Hospital of the Medical School of the Xi’an Jiaotong University. An informed consent was obtained from all participants. The diagnosis and classification were made according to the standard adopted during the National Epidemic Hemorrhagic Fever Symposium held in Nanjing in 1986. From the day the patients were admitted to the hospitals, blood and urine samples were collected twice a day, and a total of 195 blood samples and 186 urine samples were obtained, which were stored at negative 20°C. The control group consisted of 20 healthy blood donors. Their HFRS specific IgM and IgG antibodies and hepatitis virus infection indicators were all negative.
ReagentsIL-6, TNF, IL-8 monoclonal antibodies, standard samples, and negative controls were provided by Professor Jin Boquan from the Department of Immunological Teaching and Research of the Fourth Military University. Fluorescent splits were made by the Shanxi Preventive Medicine Research Medical Institute. Fluorescent indicators (isosulfocyanic acid) containing IgG and IgM extracted from sheep blood and β2-MG kits were provided by the Shanghai Vaccine and Serum Institute.
MethodsTotal serum globulins were determined by biuret. Specific IgM and IgG were detected by indirect immune fluorescence,4 and B2-MG by specific radiation fluorescence, according to the specifications of the kits. IL-6, IL-8, and TNF were determined by ELISA. The ELISA plate (96-well plate for ELISA reaction; Maxisorp, Denmark) was coated with 100μL/well of diluted capture antibody, incubated at 4°C for 48hours. On the third day, the plate was removed from 4°C and rinsed three times with wash buffer, for 5min each wash step. The standard curve and samples were added with 100μL/well and incubated at 37°C for 2h. After the plate was washed three times, diluted biotinylated detection antibodies were dripped with 100μL/well, and incubated at 37°C for 1h. The plate was washed three times. ABTS was added with 100μL/well, and incubated at 37°C for 30min. The absorption value (OD 410nm) was read by microplate spectrophotometer.
Statistical analysisResults were expressed by the mean and standard deviation. Sample mean comparison was made using Student's t-test, square analysis and linear relative analysis. A p-value <0.05 was considered significant.
ResultsCharacteristics of the participantsA total of 76 unrelated Chinese Han subjects (56 inpatients with HFRS, and 20 healthy control individuals) were included in the study. The patients were an average of 36.8 (18-61) years old, and consisted of 46 males and 10 females. The healthy controls had an average age of 34.9 (24∼55) years old, and 50% of them were males. All of the patients detected by RT-PCR were positive for viral RNA. In accordance with the 1986 Nanjing standard, ten cases were diagnosed as mild, 20 as moderate, 18 as severe, and eight as very severe. One of the very severe patients died of a lung infection, and the others survived after treatment.
Change of serum IL-6In the fever stage, the concentration of serum IL-6 in patients with HFRS was higher than that in control group (p<0.001). The concentration of serum IL-6 continued to increase during hypotension stage, reached its peak during the oliguria stage, decreased significantly during the polyuria stage, and remained higher than the controls during the convalescent stage. Fifteen patients with HFRS were tested at the same time and showed the same pattern of change (Table 1).
Dynamic change of serum IL-6 in patients with HFRS during each stage (x¯±s).
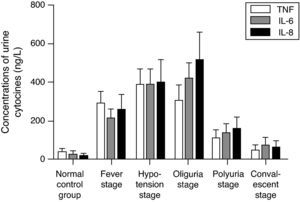
As shown in Fig. 1, the concentration of urine TNF in patients with HFRS increased remarkably during the fever stage, reached its peak during the hypotension stage, decreased during the oliguria stage, and declined significantly during the polyuria stage. It also showed that the concentrations of IL-6 and IL-8 increased during the fever stage, and continued to increase during the hypotension stage, reaching their peak during the oliguria stage. Therefore, serum IL-6 and urine IL-6 were parallel (r=0.76, p<0.01).
Relationships between the urine TNF, IL-6 and IL-8With increasing severity of the disease, the concentrations of urine TNF, IL-6, and IL-8 in patients with HFRS increased. The concentration of TNF differed significantly in each type. Except in severe and dangerous types, both the concentrations of IL-6 and IL-8 differed significantly in other types (p<0.05). IL-6 was positively related to both TNF and IL-8 (r=0.5609, p<0.005; r=0.3794, p<0.01, respectively) (Table 2).
Concentrations of urine IL-6, IL-8, and TNF in patients with HFRS in each type (x¯±s).
| Type | Number of cases | TNF (ng.L−1) | IL-6 (ng.L−1) | IL-8 (ng.L−1) | ||||
|---|---|---|---|---|---|---|---|---|
| Fever stage | Hypotension stage | Oliguria stage | Polyuria stage | Convalescent stage | ||||
| Mild | 5 | 0 | 0 | 8 | 3 | 126±53 | 147±58 | 187±69 |
| Moderate | 15 | 10 | 25 | 12 | 6 | 266±87 | 301±103 | 382±108 |
| Severe | 12 | 12 | 20 | 17 | 9 | 329±95 | 411±102 | 482±123 |
| Very severe | 5 | 5 | 11 | 7 | 4 | 411±114 | 439±136 | 539±16 |
During the course of HFRS, the concentration of IL-6 increased on the third day, peaked on the sixth day, and decreased significantly during the eighth and 12th day. The concentrations of IgM and IgG increased on the third day, and peaked on the ninth and 11th days, respectively. From the ninth day onwards, the concentration of IgM decreased significantly. The concentration of IgG remained high. The concentration of IL-6 was positively related to IgM decrease (r=0.42, p<0.05).
Relationships between IL-6 and serum globulinsSerum globulins increased during the fever stage and peaked during the oliguria stage. Serum globulins were positively related to IL-6 (r=0.51, p<0.05).
Relationships between serum IL-6 and β2-MG, BUN and Cr116 samples collected during the fever, hypotension, and oliguria stages were analyzed. The analysis demonstrated that serum IL-6 was positively related to β2-MG, BUN, and Cr (r=0.56, 0.46, 0.61, respectively). During the fever, hypotension, and oliguria stages, IL-6 was positively related to β2-MG (r=0.53, 0.56, 0.67; p<0.01).
Relationships between IL-6, and IgG and β2-MGAs shown in Table 3, with increasing severity of the disease, the concentration of IL-6 increased. The concentration in mild type differed greatly from that in moderate type, and also from those in severe and very severe types (p<0.005). The concentrations of β2-MG and specific IgG differed significantly in each type (p<0.01∼0.005). The three were highly related. The relative coefficients between IL-6 and β2-MG were 0.60, 0.66, 0.63, and 0.61 in mild, moderate, severe, and very severe, respectively. The relative coefficients between IL-6 and IgG were 0.58, 0.50, 0.55, and 0.52 in mild, moderate, severe, and very severe types respectively. The relative coefficients between β2-MG and IgG were 0.54, 0.5816, 0.60 and 0.56 in mild, moderate, severe, and very severe types respectively.
DiscussionIL-6, IL-8, and TNF are generated by multiple cells. IL-6 has immunomodulatory functions, such as differentiating B-cells to generate antibodies. The primary function of IL-8 is the induction of chemotaxis in its target cells. TNF can cause local inflammation and multiple-organ impairments.
The study showed that, at the fever stage, the concentration of serum IL-6 in patients with HFRS was higher than that of the control group, peaking in the oliguria stage, which was in accordance with the progression of the disease. The possible reason the concentration of serum IL-6 in patients with HFRS increased at early stage is that, after the HFRS virus infects the body, it proliferates in immune cells, fibroblast cells, epidermic cells, and intercapillary cells. It activates these cells, enabling them to generate more IL-6.5 At the oliguria stage, hepatic and renal dysfunction can cause difficulty in eliminating and excreting IL-6, leading to the peak of serum IL-6 concentration in patients with HFRS.
The concentrations of urine IL-6, IL-8, and TNF increased significantly at the fever stage. The concentration of TNF peaked in the hypotension stage, and decreased from the oliguria stage onwards. The concentrations of IL-6 and IL-8 peaked in the oliguria stage, and decreased significantly in the polyuria stage. The concentration of serum IL-6 was positively related to that of urine IL-6. The possible reason for the increase of urine cellular factors is that serum cytokines increased and were more excreted in the urine.6,7 After the HFRS virus infects the body, it proliferates in mesangial cells, tubular epithelium cells, and interstitial cells, causing an increase of activated cells that can produce cellular factors. The antibodies to HFRS virus could be detected in mononuclear cells, neutrophils, epidermic cells, and multinucleated giant cells, all of which had a greater ability to produce cellular factors. In renal tubules, hyperactive TNF could cause local inflammation, which is related to immune impairments of renal function. IL-6 and IL-8 participate in the formation of immune complexes at the base membrane of the renal glomerulus and in the invasion of inflammatory cells in liver. It can be concluded that immune impairments mediated by IL-6, IL-8, and TNF play an important role in renal impairments. It can also be concluded that IL-6 was positively related to IL-8 and TNF, which showed that the three might derive from the same cell.8
The concentrations of serum IL-6 and urine IL-6, IL-8, and TNF in HFRS patients increased with the increasing severity of the disease, and differed significantly in each type. This phenomenon showed that the concentrations of IL-6, IL-8, and TNF were positively related to clinical type, so determination of the concentrations of serum IL-6 and urine IL-6, IL-8, and TNF can help predict prognosis and outcome of the patients.
In each type, IL-6 was positively related to IgG, demonstrating that IL-6 was related to hyperactivity of humoral immune reaction, leading to immune-mediated pathological damages. On the sixth day, the concentration of IL-6 reached its peak. On the ninth and 11th days, the concentrations of IgM and IgG reached their peaks, respectively. The data indicate that hyperactive IL-6 was closely related to the increase of lymphocytes, to atypical lymphocytes (at different stages of development) in patients’ blood, and to that of plasma cell in marrow.9 The data also implied that the change of IL-6 was in accordance with that of plasma cells in blood and marrow, and the increased production of IgG and IgM antibodies. Therefore, IL-6 was closely related to the increase of specific antibodies during the course of HFRS. The positive relationship between the concentration of IL-6 and that of blood globulins showed that the number of immune globulins generated by β-cells significantly depended on the concentration of IL-6. β2-MG, an ideal and specific indicator of renal failure index, is inversely related to RGI.10 IL-6 was positively related to β2-MG, Bun, and Cr. During the first three stages, IL-6 was positively related to β2-MG. In each type, IL-6 was also positively related to β2-MG. These facts demonstrate that IL-6 participated in renal immune-mediated impairment, leading to the change of RGI and renal function.
Conflict of interestAll authors declare to have no conflict of interest.